Infection Prevention and Control
- Infection Prevention and Control/Surface Decontamination
- Infection Prevention and Control/Air Disinfection
List of abbreviations
| CSSD | Central Sterile Services Department |
| GNS | Guidelines, Norms and Standards |
| HEPA | High-efficiency particulate Air |
| HVAC | Heating, ventilation and air conditioning |
| ICC | Infection Control Committee |
| ICT | Infection Control Team |
| INICC | International Nosocomial Infection Control Consortium |
| IPC | Infection prevention and control |
| IPT | Isoniazid preventive therapy |
| MSDS | Material safety data sheet |
| PAC | Portable air cleaners |
| PPE | Personal protective equipment |
| SUD | Single-use device |
| TB | Tuberculosis |
| UVGI | Ultraviolet germicidal irradiation |
| WHO | World Health Organisation |
IUSS: Guidelines, Norms and Standards (GNS) reference documents
| CLINICAL SERVICES | Essential | Recommended | SUPPORT SERVICES | Essential | Recommended | HEALTHCARE ENVIRONMENT/ CROSS-CUTTING ISSUES | Essential | Recommended | PROCUREMENT AND OPERATION | Essential | Recommended |
| Adult inpatient services | X | Administration and related services | Generic room requirements | X | Integrated infrastructure planning | ||||||
| Clinical diagnostic laboratory guidelines | General hospital support services | Hospital design principles | Briefing manual | ||||||||
| Mental health | Catering services for hospitals | Building engineering services | X | Space guidelines | |||||||
| Adult critical care | Laundry and linen department | Environment and sustainability | Cost guidelines | ||||||||
| Emergency centres | Hospital mortuary services | Materials and finishes | X | Procurement | |||||||
| Maternity care facilities | Nursing education institutions | Future healthcare environments | Commissioning health facilities | X | |||||||
| Adult oncology facilities | Health facility residential | Healthcare technology | Maintenance | X | |||||||
| Outpatient facilities | Central sterile service department | X | Inclusive environments | Decommissioning | X | ||||||
| Paediatrics and neonatal facilities | Training and resource centre | Infection prevention and control | Capacity development | ||||||||
| Pharmacy | Waste disposal | X | Information technology and infrastructure | ||||||||
| Primary healthcare facilities | Regulations | ||||||||||
| Diagnostic radiology | |||||||||||
| Adult physical rehabilitation | |||||||||||
| Adult post-acute services | |||||||||||
| Facilities for surgical procedures | X | ||||||||||
| TB services | X |
PART A- POLICY AND SERVICE CONTEXT
Overview
Infection prevention and control in healthcare facilities has been recognised as a national priority area Administration, building design and engineering have rules to play in infection prevention, both fundamentally and in the supportive sense.
Healthcare facilities frequently become focal points for the transmission of communicable diseases because they bring infectious and susceptible individuals together. International research suggests that about 10% of hospital admissions result in healthcare-associated infections, which were neither present nor incubating at the time of admission. A surveillance study in developing countries conducted by the International Nosocomial Infection Control Consortium (INICC) between 2003 and 2008 revealed an unadjusted excess mortality rate from device-related infection at between 23% and 29% .
Healthcare workers are at higher exposure to infection risk through, for example, occupationally acquired tuberculosis (TB) and needle-stick injuries when compared to the background population. In South Africa, there is a scarcity of data on the prevalence of healthcare-associated infection, but general studies show that these figures are higher in developing countries[1].
The discipline of infection prevention and control (IPC) has several implications for the design and engineering of the built environment. These implications affect the enabling of standard precautions and infection control practices (such as the provision of hand basins etc.) and air quality control (for airborne infection), cohort arrangements (spatial separations) and material selection and detailing.
In healthcare facilities, IPC has been recognized as a healthcare priority and is described in subsection 2.6 of the National core standards for health establishments in South Africa .
IPC practices must be tailored to the individual facility and should be determined upfront during the design development process while being proactively addressed using an evidence-based body of knowledge. Late or retrospective consideration of IPC may result in less effective solutions, increased exposure to risk, and additional expense.
- Facilities, equipment and procedures to address standard precautions and transmission-based measures
- Curtailing cross-infection to all building users, including healthcare workers, patients and visitors
- Infection control practices in special situations
This document is intended for use by persons compiling clinical briefs for building projects, for built environment professionals and authors of infection control policies at the facility. The importance of proactive collaboration between the infection control teams, the built environment professionals and clinical service planners in developing the facility design brief and commissioning plans cannot be over-stressed. If the infection control team is not proactively involved in the earliest facility planning stages, a critical opportunity to add value will be lost.
Principles of healthcare-associated infection
Chain of infection
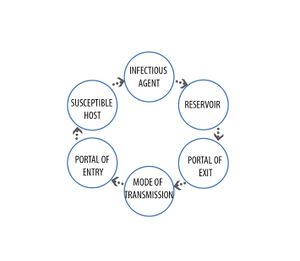
Infection is the result of contact between a viable infectious agent and a susceptible host under a suitable environment. The ability of the infectious agent to infect the host is dependent on the following characteristics of the agent:
- Viability
- Virulence
- Pathogenicity
- Dose
- Infectivity
A chain of infection involves a series of essential interdependent elements that must be in place for any transmission and infection to occur. Breaking this chain of infection at any point is generally the most effective method for preventing healthcare-associated infections
The infectious agent is the pathogen that causes disease.
A reservoir is a place where the agent can survive, for example, infected hosts or contaminated equipment.
Portal of exit is the path by which the agent leaves the reservoir, for example, gastrointestinal tract, respiratory tract.
Portal of entry is the path by which the agent enters the host, for example, gastrointestinal tract, respiratory tract and skin/mucous membranes.
Mode of transmission is the method by which the agent travels between the portals of exit and entry.
Modes of transmission
Contact transmission
Contact transmission is the most frequent form of transmission of healthcare-associated infection internationally. It can be divided into three groups, namely direct contact transmission, indirect contact transmission and droplet spread.
Direct contact transmission occurs when the physical transmission of pathogens is transferred when direct surface-of-body to surface-of-body contact occurs between a susceptible host and an infected person. This can happen when a nurse handles a patient in the course of her duties or even through patient-to-patient contact.
Indirect contact transmission is similar to direct contact transmission with the additional involvement of an intermediate object. Such an object could include medical equipment or building elements.
Droplet transmission occurs when droplets are generated and ejected from an infected host through talking, sneezing or coughing. When these droplets have sufficient size and mass, they retain their ability to carry viable pathogens to a susceptible host, but the mass of these particles normally limits the distance they can travel to less than approximately 1 m. When these droplets settle on a suitable site on a susceptible host, the host can be infected. Certain pathogens are limited to this type of transmission, as they do not remain viable on smaller particles such as droplet nuclei.
Airborne transmission
When particles expelled from an infected host are so tiny that they evaporate leaving a droplet nuclei (<µ5m) containing pathogens, these pathogens can be carried about on air currents. These may remain airborne for hours, which allows them to travel long distances from the infectious host. When inhaled, viable organisms embedded in the droplet nuclei may cause infection.
TB is of primary concern in South Africa. Together with HIV/Aids, it is the greatest cause of morbidity in adults. In South Africa, it is also the fourth highest cause of death in infants and has caused an average life expectancy reduction of 10 years within the last 10 years. Drug-resistant strains of TB can also be contracted by inhalation of viable droplet nuclei. These strains are more expensive and difficult to treat than susceptible strains with poorer outcomes. TB is exclusively spread by airborne transmission. Other airborne diseases include chickenpox (varicella) and measles (rubella).
Vector transmission
Vector-borne diseases play a minor role in healthcare-associated infections and require the assistance of vectors such as rats, mosquitos and flies to transmit microorganisms. Transmission can be through simple contact with a contaminated vector or its waste. Vectors can also transmit micro-organisms through actual penetration of the skin or other membranes by bites or burrowing.
Vehicle transmission
Vehicle transmission occurs when pathogens are transmitted by contaminated sources such as food, water, medication or equipment. Vehicle transmission relies on the mechanisms described under contact transmission.
PART - Infection Prevention and Control Principles
Overview
Infection control practices can be categorised into two categories. The first category is standard precautions, which must be applied at all times. The second category is additional transmission-based controls where, under particular circumstances, such as patient diagnosis, infectiousness, vulnerability profiles of susceptible individuals or epidemiological factors, additional precautions are indicated.
IPC is practised in a systematic, hierarchical bundle, where combinations of evidenced-based mitigation measures are taken to address risk. While the principles may remain the same, the actual mitigation measures would differ between service type and agent risk under consideration.
Standard precautions
Standard precautions include administrative, environmental (engineering) and personal protective controls as described in the elements listed below.
IPC practice is a systematic hierarchical bundle of mitigating measures for managerial, administrative, environmental and personal protective risk management.
Infection control programme
The facility should develop an IPC policy that is consistent with the national core standards for health establishments in South Africa, as amended (South Africa, 2011). The elements of such a programme should include the following:
- A surveillance plan that identifies critical control points, monitoring intervals and acceptance limits
- A responsibility matrix that identifies staff, teams, responsibilities, requisite skills, required training and training plans
- The identification of available products and services that are essential for maintaining safety and hygiene and determine usage volumes
- Associated protocols for surveillance, training, hygiene and safety.
The infection control committee and teams
According to the infection prevention control policy of South Africa, all healthcare facilities should have an IPC focal person. It is recommended that an infection control committee (ICC) be established in each healthcare facility to facilitate multidisciplinary input and provide resources to the infection control team (ICT). The ICC membership should reflect the spectrum of clinical and administrative services being offered at that facility. The committee should ideally include a microbiologist, an infection control nurse and doctor, an occupational health practitioner, representatives from major clinical specialities, and maintenance, administrative and representatives from other departments as deemed necessary. The ICC is responsible for ensuring that the facility has a facility-specific, coherent IPC plan that aligns with the national strategy and that this plan is documented and maintained in the IPC manual.
The ICC should hold regular meetings in which minutes are kept. These minutes should be copied to the facility’s management committee, as well as any departments directly involved in the proceedings and discussions of the meetings.
The ICTs are responsible for the day-to-day running of the IPC programmes and should consist of at least one physician, one microbiologist and one infection control officer. Each facility should have at least one ICT that is accessible for infection control advice on a 24-hour basis. The ICT should meet regularly enough to discuss daily incidents and issues and should have a standing agenda to discuss IPC surveillance, policy and practice, communication and training, and problems. Minutes should be prepared for these meetings and kept on file. The ICT is responsible for updating and maintaining the IPC manual with approval from the ICC.
Hand hygiene
Hand hygiene is a highly effective method for combating direct and indirect contact transmission. Hand hygiene is performed by washing hands with soap and water or by rubbing the hands with an antimicrobial agent. The World Health Organization recommends a 75% volume/volume (v/v) isopropanol or 80% v/v ethanol low-viscosity hand rub (World Health Organization, 2010). The adoption of alternative solutions, such as gels and foams, will require evidence of efficacy or thorough validation.
In facilities where the use of existing hand basins are replaced by alcohol hand rubs, the increased risk of legionella breeding in and spreading from disused hand basins needs to be mitigated.
Clinical hand basins have the following requirements:
- Low-flow aerosolising faucets are not allowed, as these promote the droplet spread of possible waterborne pathogens like legionella.
- Elbow-operated taps are preferable over electronically activated taps as the latter presents additional maintenance and reliability issues and unit failure could undermine the hand hygiene programme.
- Water stream from faucets should not fall directly into the waste outlet to prevent the possible aerosolisation of pathogens residing in the waste outlet.
- Hand basins should not include waste overflows, as these provide an additional risk for aerosolisation of pathogens breeding in the waste pipes.
The reader is directed to the Building Engineering Services and the Generic Room Requirements guidance documents for additional information.
Aseptic techniques
Aseptic techniques can make special demands on facility and space planning. An example of this is the scrub procedure of surgical staff and movement protocol between the scrub room and the operating theatre. In this instance, the scrub team clasp their hands and raise their elbows as they move into the theatre to prevent contamination after scrubbing. This action would be hindered by conventional doors, door mechanisms and door sizes. Sliding doors and wider openings need to be considered in this instance.
The reader is directed to the Facilities for Surgical Procedures and the Generic Room Requirements guidance documents for further information.
Barrier nursing
When nursing care is given to patients who are suffering from highly infectious diseases, are severely immuno-compromised or highly susceptible to infection, barrier nursing techniques are used. Nursing staff use agent-appropriate masks, respirators, gowns and gloves to provide the requisite protection. Also, barrier nursing may require containment or protective isolation facilities. Barrier nursing can combat contact and airborne transmission. For additional information on the requirements on facilities for barrier nursing and isolation, the reader is directed to the Adult Inpatient Services guidance document.
Housekeeping and cleaning
Cleaning, disinfection and sterilisation are central to any successful IPC plan. This holds for facilities cleaning, hand hygiene and instrument sterilisation.
Environmental surfaces, such as those in patient-rooms and theatres, are classified as ‘non-critical’ according to the Spaulding system, which classifies objects based on their ability to spread infection. This classification applies to medical devices but does not imply that these surfaces should not be subject to hygienic design principles and rigorous cleaning, even though they may not require regular disinfection. However, high-touch areas should be identified and these should be decontaminated regularly to prevent contact transmission. These surfaces should be durable and resilient to the available cleaning, disinfection and sterilisation methods and chemicals employed.
The safety and efficacy of disinfectants and detergents must be established before they are adopted for use. Material safety data sheets must be available and reviewed for all cleaning materials. Special precautions and protocols must be adopted, maintained and monitored where required. Appropriate and available disinfectants and detergents should be identified, reviewed, quantified, budgeted for and documented in the IPC manual.
Disposal of waste disinfectants and detergents should be per the national regulations and the Waste Disposal and Decommissioning articles.
Materials and finishes
The primary concern relating to materials and finishes in combating contact transmission is that these should be selected and installed so that they are cleanable, durable, non-particle-liberating and impervious in clinical areas. Material selection for IPC should be limited to settings that warrant it. For example, antimicrobial surfaces can be used on high-touch areas of general patient areas (for example, door handles) and all surfaces in burn units.
For more information on this extensive and complex subject, the reader is directed to the Materials and Finishes article.
Waste management
For information on IPC-related topics, such as sharps disposal, spill management, equipment, and material handling and classification, the reader is directed to the Waste Disposal and Decommissioning articles.
Personal protective equipment
The following personal protective equipment (PPE) should be considered with standard precautions against contact and airborne transmission:
- Gloves
- Caps
- Gowns
- Masks
- Respirators
- Overshoes (critical areas)
In their designs, facility designers should make provision for the secure storage consumables, the appropriate day-to-day access to consumables and their routine disposal and/or decontamination. These items should be identified, reviewed, quantified, budgeted for and documented in the IPC manual.
Zones
Healthcare facilities can consist of zones of varying risk of infection transmission. These areas can be described as non-critical, critical or aseptic. Environmental IPC in these areas may be achieved through the implementation of primary and/or secondary protective barriers.
To identify these areas and their associated risks and protocols to staff, patients and visitors, the colour-coding of building elements should be implemented, and clear signage should be included. For areas of exceptional infection risk, access control systems should be implemented.
High-risk areas
Areas presenting a high risk of infection transmission include, but are not limited to the following:
| Area | Patient At Risk | Staff at Risk |
|---|---|---|
| Theatres | X | X |
| Central Sterile Services Department (CSSD) | X | |
| Burns | X | X |
| Bone marrow transplant | X | |
| ICU | X | |
| Isolation – containment | X | |
| Isolation – protective | X | |
| Oncology | X | |
| Dialysis | X | |
| Laboratories | X | |
| Sluice rooms | X | |
| Plant rooms (filters, wet-services and ducting) | X | X |
| Indoor Waiting Areas | X | X |
An appropriate level of response in developing administrative environmental controls to mitigate the risks in these areas is important. The reader is directed to the Airborne Precaution Risk Classification Matrix proposed in the Building Engineering Services guidance article and the discussion of isolation rooms in the Adult Inpatient Services guidance article for further information
Pest control
A pest control policy must be implemented to combat vector transmission. It is common practice to contract pest control services. Even when this service is outsourced, a record of the number of vermin encountered and exterminated should be collected and maintained to assess the effectiveness of the pest control programme. These records must be maintained and reviewed by the ICT.
Important elements in a pest control programme include the following:
- Baiting and trapping on the facility perimeter
- Waste management practice that does not create an attraction for pests
- Screening of ventilation, window and access openings where vector and vehicle transmission presents a high transmission risk (kitchens, plant rooms, wards in high malaria-risk areas)
- Bird repellents for enclosed and semi-enclosed areas that attract birds
Maintenance
Without effective planned maintenance, any IPC plan could not be truly effective. Maintenance programmes and considerations that directly affect the IPC outcomes include the following:
- Legionella control programme
- Filter maintenance
- Plant room and service space hygiene
- The in-house stock of critical spare parts (fan belts, filters, breakers etc.)
Additional transmission-based controls
Airborne contamination control
In the context of high tuberculosis (TB) burden in resource-constrained settings, an effective airborne contamination control policy is critical in healthcare facilities.
A variety of control measures are available to break the chain of TB infection. These measures can be grouped into administrative controls, environmental controls and personal protective controls.
The World Health Organization (WHO) describes the elements of a hierarchy of controls (World Health Organization, 2019). Administrative controls are awarded the greatest impact, while personal respiratory protection the least weight of the three.

Administrative controls
Healthcare facilities need to develop a policy and action plan to guide their TB infection control programmes. A committee should be formed and a leader appointed in writing. Members of the committee should be appointed in writing and the terms of reference developed.
An annual risk assessment should be conducted to understand the risk of transmission throughout a health facility. The results of the risk assessment should inform the development of risk management plans and corrective actions taken.
Essential elements of a good TB infection control programme should include the following:
- Training of healthcare workers
- Health education for patients
- Screening of patients for TB symptoms
- Triaging, separation and fast-tracking of patients
- Provision of Isoniazid Preventive Therapy (IPT) for eligible patients
- Cough etiquette and provision of surgical masks
- Screening of healthcare workers and the provision of voluntary counselling and testing (VCT)
- Provision of IPT to eligible healthcare workers
Environmental controls
Essential elements of a good programme for environmental control should include the following:
- There should be outside sputum collection points or sputum collection booths for internal use.
- Overcrowded and cramped waiting areas should be avoided.
- Waiting in passages should be prohibited.
- Outside waiting areas should be created to reduce overcrowding.
- To optimise natural ventilation, windows with large opening parts should be installed. The opening parts should be at least 20% of the floor area.
- Where mechanical ventilation is installed in high-risk areas, airflow rates should be equal to the greater of 12 air changes per hour or 80 litres per second per person.
- These mechanical systems should be maintained regularly and a maintenance log should be kept.
- Where ultraviolet germicidal irradiation is used, installation should be supervised by a competent person. The efficacy of installations should meet recognised standards and should be properly maintained and a maintenance log kept.
The reader is directed to the Building Engineering Services guidance document for additional guidance on environmental controls.
Personal respiratory protection
The development of a good respiratory protection programme is very important for health facilities. This programme should include the following:
- Medical evaluation of staff to check if they are fit to use respirators.
- Choice of respirators should meet international standards.
- Training of staff in respirator use.
- Fit testing to determine which sizes of respirators to procure for staff.
The reader is directed to the TB Services article and the Implementing the WHO Policy on TB Infection Control in Healthcare Facilities, Congregate Settings and Households guidance documents for additional guidance on PPE usage and care.
Ultraviolet germicidal irradiation
Ultraviolet germicidal irradiation (UVGI) has been effective in disinfecting contaminated water, air and surfaces, but it should be considered a supplement to other infection control measures. UVGI has several proven use-cases. However, in each case, maintenance, system design and correct protocols are significant hurdles to cross in terms of the broad adoption of the technology.
Water disinfection
UVGI is an effective localised method of disinfecting water streams. As UVGI is not dispersive, any contaminant sources or reservoirs downstream of the point of disinfection are unaffected by the system. Monitoring and maintenance of water-system UVGI installations are quite onerous and challenging.
Air distribution ducting
The disinfection of air streams in air distribution ducting can be quite effective when viewed in isolation. However, when considered a part of a larger regulated system, the application of in-duct UVGI is quite limited. In most applications that require the requisite sterility in the supply, air quality would need the additional particle filtration offered by a high-efficiency filter (HEPA). Where HEPA filters are installed, in-duct UVGI offers no real additional advantage. The same argument applies to exhaust air treatment, where UVGI cannot offer the same level of confidence, redundancy, and particle and odour control as regular air filtration.
Air-handling units and cooling coils disinfection
UVGI has proven effective in reducing the cleaning maintenance required on heating, ventilation and air conditioning (HVAC) cooling coils. In these applications, UVGI should be used in association with course air filtration. (ASHRAE 2013)
Air-handling units and cooling coils disinfection
UVGI has proven effective in reducing the cleaning maintenance required on heating, ventilation and air conditioning (HVAC) cooling coils. In these applications, UVGI should be used in association with course air filtration. (ASHRAE 2013)
Fumigation and decontamination
Gaseous fumigation is an effective and universal method for space and surface disinfection. It is important when planning and designing healthcare spaces to consider whether an area would be subject to gaseous fumigation. Where this is a requirement, careful planning should be undertaken to ensure the correct technology could be adopted. The following aspects should be considered when selecting a fumigation technology for a considered space:
- Safety – Many fumigation solutions are highly noxious, toxic, corrosive or carcinogenic. The proximity and permeability of the considered space to sensitive areas and equipment should be considered.
- Effectivity – Fumigant effectiveness varies between fumigant and agent combinations. At the planning stage, it is helpful to understand the agents that are targeted, as well as the operational time that is required and available for fumigation activities.
- Cost – Cost becomes a significant planning factor when considering customised installations, exclusive technologies or registered trademarks. Also, many of the newer technologies offer significant improvements in effectiveness and safety at a significant additional cost.
When selecting a fumigation technology, the confluence of safety, life cycle cost, service and pathogen-specific decontamination effectiveness needs to be taken into account.
Terminal decontamination plans need to be developed and implemented for rooms used by highly infectious patients.
Sterilisation equipment should be licensed and obtained from an approved supplier with a manual, service plan and service record as required.
Refer to Infection Prevention and Control/Surface Decontamination for detailed information on surface decontamination.
Filtration
HEPA filtration assists ventilation systems in reducing airborne transmission by preventing the recirculation of airborne contaminants. This can be achieved within the central HVAC system or through portable air cleaners (PACs).
PACs can, in theory, aid in the removal of airborne contaminants if they are sufficiently sized and appropriately designed. The reader should be warned that the majority of systems that are commercially available at a reasonable cost compared to heating ventilation and air-conditioning (HVAC) based filtration systems have not yet been proven to offer significant effectiveness against airborne transmission.
- Sizing - for PACs to offer adequate performance, they should reasonably be equivalently sized to regular fan-filter systems. Since these systems are seldom designed with the considered room’s ideal ventilation effectiveness in mind, they may even need to be larger than that required from a central HVAC system to achieve equivalent results.
- Efficacy studies for PACs are inconclusive.
PART C - Definitions
| Adventitious contamination | The accidental introduction of environmental micro-organisms onto a medical device or product. |
| Anatomy | The study of the structure of living things. |
| Antimicrobial | The ability of a process or product to be effective against micro-organisms by either killing them or inhibiting their growth. |
| Antisepsis | Destruction or inhibition of micro-organisms in or on living tissue, for example, skin, mucous membranes or wounds. |
| Antiseptic | An antimicrobial product or process used on the skin or other living tissues. In some countries, antiseptics are labelled as disinfectants or antiseptic disinfectants. |
| Asepsis/Aseptic | Free of micro-organisms or using methods to keep free of micro-organisms. |
| Bacteria | Also known as eubacteria. A group of unicellular, prokaryotic micro-organisms. Examples include Bacillus, Staphylococcus, and Pseudomonas. |
| Biocide | A general term referring to any chemical or physical antimicrobial agent that inhibits or inactivates life. The term ‘micro-biocide’ are those biocides that are effective against micro-organisms. Chemical biocides include chlorine, iodine, alcohol and hydrogen peroxide. Physical biocides include heat and radiation. |
| Biofilm | Communities of micro-organisms (either singular or multiple species) developed on or associated with surfaces. |
| Carrier | A device that carries, conveys or transports the load through the department via a trolley/carriage or on a rail/track into/through a decontamination process. |
| Cleaning chemistry | A formulation (or mixture of chemicals) designed for cleaning purposes. Cleaning chemistries are often referred to as ‘detergents’, but detergents are usually only one part of these mixtures and can include biocides, enzymes, buffers, chelating agents and other components. |
| Cleaning | The removal of contamination (often referred to as ‘soil’) from a surface to the extent necessary for further processing (e.g. disinfection and sterilisation) or for intended use. |
| Contamination | The presence of dirt or ‘soil’ that can include various materials, forms of chemistry, and bioburdens (such as micro-organisms). Depending on the situation, contamination may be visible (e.g. a blood spill) or invisible (e.g. the presence of micro-organisms). Contamination of a device (e.g. an endoscope) following patient use is generally referred to as ‘soil’. |
| Decontamination | Physical and/or chemical means to render a surface or item safe for handling, use or disposal. In many cases, decontamination is at least a two- or even three-step process to include cleaning and disinfection and/or sterilisation. However, cleaning alone or a multi-step process of cleaning, disinfection and sterilisation may also be required for decontamination, depending on the final use of the surface/item. |
| Detergent | A compound, or a mixture of compounds, intended to assist cleaning. Detergents are a sub-class of surface-active agents (‘surfactants’). They are commonly also referred to as cleaners or cleaning chemistries, but detergents are only one part of these mixtures. |
| Device | Any instrument, apparatus, appliance, material or other article intended for the purpose of diagnosis, prevention, monitoring, treatment or alleviation of disease or other medical/surgical use. A reusable device is designed for use many times on different patients. The device comes with detailed instructions on how it can be safely reprocessed between each patient. A single-use device (SUD) has been designed by a manufacturer to be used on a single patient only and then discarded. The terms ‘device’ and ‘instrument’ are used interchangeably throughout the book. |
| Disinfectant | A physical or chemical product for disinfection. |
| Disinfection | The antimicrobial reduction of the number of viable micro-organisms on a product or surface to a level previously specified as appropriate for its intended further handling or use. There are various levels of disinfection, based on their ability to kill certain types of micro-organisms. |
| Facility | A complex of buildings, structures, roads and associated equipment, such as a hospital or healthcare facility that represents a single management unit for financial, operational maintenance or other purposes. |
| Faucet | A device for controlling the flow of a liquid; normally from a pipe |
| Fixtures | Items that are permanently fixed to the building or permanently connected to a service distribution system. These items require service connection (e.g. electrical, hydraulic and mechanical) and includes basins, light fittings, clocks and medical service panels. |
| Fittings | Refers to fixed items attached to walls, floors or ceilings that do not require service connections, such as hooks, mirrors, blinds, joinery, pinning and writing boards. |
| Fumigation | The delivery of a disinfectant to an area by aerial dispersion, usually in the form of a gas, vapour or aerosol. |
| Fungi | A group of cell wall containing eukaryotic microorganisms, that can be further sub-divided into moulds (or filamentous fungi, as they can form long filaments or lines of cells) and yeast (unicellular, single-celled forms). |
| Gross area | Gross area includes, in addition to the net area, partitions and circulation internal to the functional area of the department. |
| Guideline | Document used to communicate recommended procedures, processes or usage of particular practices. |
| Infection | The detrimental introduction and colonisation of a host (human/animal) by a micro-organism. |
| Infection prevention/control | The discipline concerned with preventing the spread of infection. Often used interchangeably, ‘prevention’ is more correctly used to describe practices of preventing infection, while ‘control’ may also include practices to control an infection after it has taken place (e.g. isolation precautions, antibiotic or other drug-based therapy). |
| Label | An identification indication on a product or other article. This can include the manufacturer, address, product/item identification (e.g. serial or part number) and instructions for use. A product label can include what is physically written on the product (the attached label), but any also included other accessory information, such as additional instructions for use, the material safety data sheets (MSDSs) and technical literature. |
| Load | Collectively, all the goods, equipment and materials that are put into a steriliser or washer/disinfector at any one time for processing. |
| Medical device | Any instrument, apparatus, appliance, material or other article. |
| Mobile equipment | Equipment items (medical or non-medical) that require electrical or mechanical connections or floor space. |
| Net area | The actual floor area in a room or functional area (finish to finish) that can be used by people, furnishings or equipment. |
| PPE (personal protective equipment) | Specialised clothing or equipment worn by an employee for protection against a hazard. |
| Pressure | The effect that occurs when a force is applied on a surface or the force applied per unit area. For example, atmospheric pressure is the pressure exerted by the earth’s atmosphere. |
| Process | A designed sequence of operations or events, possibly taking up time, space, expertise or other resource, which produces some outcome. |
| Safety | The condition of being protected from danger, harm or injury. |
| Soil | See contamination. |
| Spaulding Classification | System of categories based on the degree of the risk of infection e.g. critical, semi-critical and non-critical as devised by Earle H. Spaulding (1939) |
| Sterile | Free from viable organisms. |
| Sterile barrier system | Package that provides a barrier to micro-organisms and allows the aseptic presentation of the product at the point of use. |
| Sterilisation | A defined process used to render a surface or product free from viable organisms, including bacterial spores. |
| Tray | A container, usually with a flat base and upturned edges, used for containing an assembly of surgical instruments for packing to be used in an aseptic procedure. |
| Validation | Documented procedure for obtaining, recording and interpreting the results required to establish that a process would consistently yield a product complying with predetermined specifications. |
| Virus | A class of micro-organisms that is unable to grow or reproduce outside a host cell. |
| Washer/disinfector | A machine that cleans and disinfects medical devices and other articles used in the context of medical, dental and pharmaceutical practice. |
Part D - Notes and References
Friedman, C. and Newsom, W. 2011. IFIC basic concepts of infection control. Portadown: International Federation of Infection Control. [pdf] Available at: <http://www.theific.org/basic_concepts/IFIC%20Book.pdf> [Accessed March 2014]
National Institute for Occupational Safety and Health. 2009. Environmental control for tuberculosis: Basic upper-room ultraviolet germicidal irradiation guidelines for healthcare settings [pdf]. Atlanta: Centers for Disease Control and Prevention. Available at: <http://www.cdc.gov/niosh/docs/2009-105/> [Accessed March 2014]
Rosenthal, V.D. et al. 2009. International Nosocomial Infection Control Consortium (INICC) report, data summary for 2003-2008. American Journal of Infection Control, 38(2), pp.95–104.e2.
World Health Organization. 2009. WHO Policy on TB Infection Control in Healthcare Facilities, Congregate Settings and Households.[pdf] Geneva: World Health Organization. Available at: <http://whqlibdoc.who.int/publications/2009/9789241598323_eng.pdf> [Accessed March 2014]
South Africa. 2011. National core standards for health establishments in South Africa. National Department of Health: Pretoria.
TB-Infection Control Subgroup. 2009. Implementing the WHO Policy on TB Infection Control in Healthcare Facilities, Congregate Settings and Households.[pdf] Available at: <http://www.stoptb.org/wg/tb_hiv/assets/documents/TBICImplementationFramework1288971813.pdf> [Accessed March 2014]
World Health Organization. 2010. Guide to local production: WHO recommended handrub formulations, pp.1–9. [pdf]. Geneva: World Health Organization. Available at: <http://www.who.int/gpsc/5may/Guide_to_Local_Production.pdf> [Accessed March 2014]
American Society for Heating Refrigeration and Air-Conditioning Engineers, 2013. Handbook. [pdf] Atlanta: ASHRAE. Available at: <https://www.ashrae.org/resources--publications/handbook> [Accessed March 2014]
- ↑ Cite error: Invalid
<ref>tag; no text was provided for refs named:0