Legionella Control
INTRODUCTION
HISTORY

Legionnaires’ disease was first described after a pneumonia outbreak at an American Legion Convention held in Philadelphia during 1976. In total, 182 delegates were affected and 29 died before workers at the Centers for Disease Control and Prevention (CDC) based in Atlanta, USA isolated the causative organism in January 1977. The organism was placed in the family Legionellaceae, genus Legionella to commemorate the first victims of the disease. The first species was named Legionella pneumophila (Greek for “lung loving”).
It soon became clear that legionellae were not really new; retrospective studies showed that an organism isolated for the first time in 1944 (called Tatlockia micdadei) actually belonged to the genus Legionella. The first strain of Lpneumophila was isolated already in 1947 from a guinea pig that had previously been inoculated with blood from a patient with what was called an “unknown febrile disease” at the time.
The two decades following the discovery of the family Legionellaceae was marked by rapid developments in Legionella detection and the identification of numerous new species. Twenty-eight new Legionella species and two “Legionella-like amoebal pathogens” (LLAPs) (LLAP-1 and LLAP-6) were isolated during the 1980s, mostly from sources in the USA. The 1990s were marked by an increase in Legionella isolation from countries in Europe and Australia with fifteen new Legionella species being described for the first time.

More than half of the currently known Legionella species are potentially pathogenic to humans. L. pneumophila is still implicated in >80% of infections; however, as more species are isolated from environmental sources worldwide, even those species not yet associated with disease should be considered as potentially pathogenic until proven otherwise. For example, L. longbeacheae, often isolated from potting soil, is considered the most common cause of legionellosis in Australia.
Legionellae are faintly staining gram negative, rod-shaped, non acid fast bacteria that to not form spores or capsules. All species except L. oakridgensis are motile. Legionellae are typically between 0.3 and 0.9 µm wide and 1- 20 µm long. However, shorter forms measuring 1- 2 µm in length are often observed in clinical specimens or under conditions of iron deprivation.
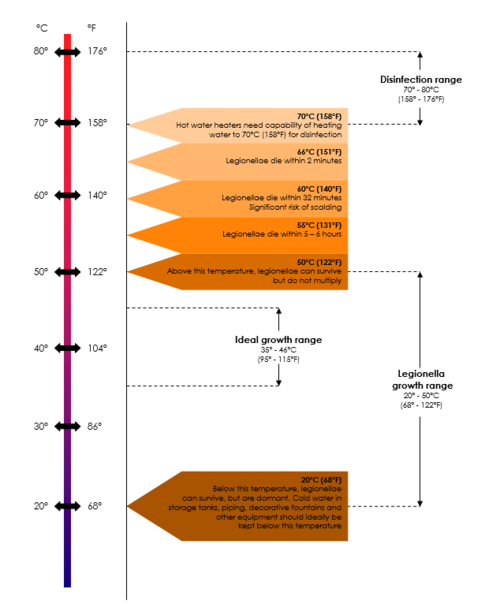
The ability of legionellae to grow in water is influenced by several factors. They can grow at temperatures between 20°C and 60°C, with optimal growth occurring between 37°C and 45°C. They prefer a pH in the range of 5.0 9.5 and only grow in the presence of Lcysteine, HCl and iron salts. Legionella-like amoebal pathogens (LLAPs) are very similar to Legionella species in that they are gram negative, infect amoebae and can survive and multiply intracellularly. However, they cannot be cultured on laboratory media. The first LLAP was isolated[1] from soil in Poland in 1954 and was named Sarcobium lyticum. The next isolation of an LLAP was in England more than 20 years later. Since then, LLAPs have been isolated from various sources, mostly associated with confirmed cases or outbreaks of [2][3] Legionnaires’ disease. Three of the LLAPs have since been reclassified as L. drozanskii, L. rowbothamii and L falloni. The currently known LLAPs are listed in the table below.
INTERACTIONS WITH PROTOZOA
Legionellae are slow-growing organisms that require a combination of nutrients for growth. Due to their fastidious nature and lack of antibiotic activity, they may be replaced by faster growing organisms if they do not have an alternative means of survival in aquatic environments. The fact that legionellae are ubiquitous in these environments suggests that protozoa, especially amoebae, play a supportive role in their survival and multiplication. In fact, their natural habitat, parasitic to protist hosts, has now been[4] proven.
| ORGANISM | SG | YEAR | SOURCE | PATHOGEN |
|---|---|---|---|---|
| L adelaidensis
L anisa L beliardensis L birminghamensis L bozemanii L brunensis L cherrii L cincinnatiensis L donaldsonii* L drozanskii (LLAP-1) L dumoffii L erythra L fairfieldensis L fallonii (LLAP-10) L feeleii L geestiana L gormanii L gratiana L gresilensis L hackeliae L israelensis L jamestowniensis L jordanis L lansingensis L londiniensis L longbeacheae L lytica L maceachernii L micdadei L moravica L nautarum L oakridgensis L parisiensis L pittsburghensis L pneumophila L quateirensis L quinlivanii L rowbothamii (LLAP-6) L rubrilucens L sainthelensi L santicrucis L shakespeari L spiritensis L steigerwaltii L taurinensis L tusconensis L wadsworthii L waltersii L worsleiensis |
1
1 1 1 2 1 1 1 * 1 1 2 1 1 2 1 1 1 1 2 1 1 1 1 1 2 1 1 1 1 1 1 1 1 15 1 2 1 1 2 1 1 1 1 1 1 1 1 1 |
1991
1985 2001 1987 1980 1989 1985 1988 2002 2001 1980 1985 1991 2001 1993 1980 1991 2002 1985 1985 1985 1982 1994 1993 1982 1996 1985 1980 1989 1993 1983 1985 1980 1979 1993 1990 2001 1985 1984 1985 1992 1985 1985 1999 1990 1983 1996 1993 1993 |
Cooling water (Adelaide Australia)
Faucet (Chicago), tap water (LA) Water, France Lung biopsy (Alabama) Lung aspirate (Toronto) Cooling tower water (Czechoslovakia) Thermally altered water (Minnesota) Lung tissue (Cincinnatti) * Tank of well water (Leeds 1981) Lung tissue Cooling tower water (Seattle) Cooling tower water (Fairfield Australia) Ship air conditioner (1994) Grinding machine coolant fluid Hot water tap, office building (London) Bronchial wash of pneumonia patient Thermal spa water (France) Water, France Bronchial biopsy (Ann Arbour) Water (Israel) Wet soil (New York) Water and sewage (Israel) Bronchial washing, hloramin patient Office building cooling tower (London) Human lung (Longbeach Australia) Previously Sarcobium lyticum Water (Phoenix) Human blood via yolk sac Cooling tower water (Czechoslovakia) Hot water tap (London) Cooling tower water (Pennsylvania) Cooling tower water (Paris) Synonym for L micdadei, strain TATLOCK Water (Pennsylvania) Shower in hotel bathroom (Portugal) Water in bus airconditioner (Australia) Water and sludge, industrial liquefier Tap water (Los Angeles) Spring water (Washington) Tap water (Virgin Islands) Cooling tower water (England) Lake water (Washington) Tap water (Virgin Islands) Water, hospital humidifier (Italy) Pleural fluid, transplant patient (Arizona) Sputum Potable water system (Australia) Industrial cooling water (England) |
Unknown
Yes Unknown Yes Yes No Yes Yes * Yes Yes No Unknown Yes Yes Unknown Yes No Unknown Yes No No Yes Yes Unknown Yes Yes Yes Yes No Unknown Yes Yes Yes Yes Unknown No Yes Yes Yes No Unknown No No Unknown Yes Yes Unknown Unknown |
| STRAIN | HOSTS | YEAR | ORIGINAL SOURCE | PATHOGENIC |
| Sarcobium lyticum | A polyphaga
H vermiformis |
1954 | Soil | Yes |
| LLAP-1 | A polyphaga | 1981 | Tank of portable water well | Yes |
| LLAP-2 | A polyphaga
H vermiformis |
1986 | Garage steam cleaning pit | Yes |
| LLAP-3 | A polyphaga | 1986 | Sputum from pneumonia patient | Yes |
| LLAP-4 | A polyphaga | 1986 | Hospital whirlpool bath | Yes |
| LLAP-5 | A polyphaga | 1988 | Nursing home plant spray | Yes |
| LLAP-6 | A polyphaga
H vermiformis |
1988 | Factory liquefier tower | Yes |
| LLAP-7 | A polyphaga
H vermiformis |
1991 | Hotel whirlpool spa | Yes |
| LLAP-8 | H vermiformis | 1990 | Hospital shower | Yes |
| LLAP-9 | A polyphaga
H vermiformis |
1992 | Factory cooling tower | Yes |
| LLAP-10 | A polyphaga | 1994 | Ship air-conditioning system | Yes |
| LLAP-11 | A polyphaga | 1993 | Furnace cooling system | Yes |
| LLAP-12 | A polyphaga | 1994 | Furnace cooling system | Yes |
Rowbotham[5] was the first to demonstrate interactions between legionellae and protozoa. To date, protozoa of the genera Acanthamoeba, Tetrahymena, Naegleria, Echinamoeba and Vanella species have been implicated in these interactions. Not much is known about the metabolic and physiological status of legionellae after passage through protozoa, but in vitro studies have shown changes in their physiological status resulting in iron deprivation, possibly changing the susceptibility of the released bacteria to chemical inactivation. Although they can survive extracellularly, this phase is believed to be only temporary while they are searching for new hosts to[6] infect. Furthermore, it was recently documented that very few Legionella bacteria are needed to start intracellular replication. Some workers have reported a 7000 times increase in Legionella colony forming units (CFU) after intracellular replication but there is no consensus yet as many believe that this intracellular replication cycle is not necessary for the proliferation of legionellae within mixed bacterial populations. From these studies it is clear that there is still extensive research to be done on this aspect of Legionella survival in the environment. Until more becomes known, it is unsafe to assume that the absence of protozoa within water samples prevents the survival of legionellae; as long as there are other bacterial species present, appropriate measures should be taken to prevent Legionella proliferation.
HOW DOES THE INTERACTION WITH PROTOZOA BENEFIT LEGIONELLAE?
Amoeba trophozoites feed and multiply in water and biofilm. When conditions become unfavourable, these trophozoites are transformed into cysts with hard, impermeable outer walls that provide protection for ingested Legionella organisms. When conditions become more favourable, the cysts change to trophozoites again and the bacteria are set free. Legionellae have been[7] recovered from cysts treated with 50 parts per million (ppm) chlorine suggesting a high level of protection by the cysts. This high resistance of amoebal cysts to biocides may be the mechanism for the apparent reseeding of water systems by legionellae often experienced in the water treatment industry. However, recontamination may also occur via transmission of airborne cysts acting as carriers for the legionellae.
HOW IMPORTANTIS LEGIONELLA IN SOUTH AFRICA?
Very little has been published on Legionella in South Africa. After the initial introduction of diagnostic laboratory tests in 1979, legionellosis cases were identified in Durban, Port Elizabeth and Johannesburg during the early 1980's. By 1982, antibodies to L.[8][9] pneumophila had been demonstrated in 10% of hospitalised pneumonia patients, a figure that was confirmed in 1994. A high[9][10][11] prevalence of antibodies was also demonstrated in workers in the mining industry and the general public. Despite this high prevalence, only one Legionnaires' disease outbreak and less than 40 sporadic cases have been reported since legionellosis became notifiable in 1990. Similarly, very little is known about the prevalence of Legionella in the South African environment. Low concentrations of[12] legionellae were reported in 77% of cooling towers in a large study reported in 1991. More recently, culturable legionellae were present in 82% of industrial water samples tested; 54% of these samples yielded legionellae in numbers equal to or in excess of 1000 11 CFU/ml3
LEGIONELLA DETECTION
Classical detection methods for Legionella species relied on the inoculation of susceptible guinea pig hosts. Although selective, these methods were expensive and time consuming and were soon replaced by isolation by culture on agar media. To improve the recovery of legionellae by culture, the use of certain selective media and steps were introduced to minimise contamination by nonlegionellae. In attempts to simplify Legionella identification, radioimmunoassays (RIAs), enzyme linked immunosorbent assays (ELISAs), agglutination tests and nucleic acid probes and polymerase chain reaction (PCR)-based assays have since been developed and tested.
Despite the relative success of these new methods for the detection of environmental legionellae, culture remains the method of choice. However, no single culture method has so far proven to be ideal for all samples in all given circumstances and environments. Even in the absence of contaminating bacteria or other inhibitory substances, the detection of small numbers of legionellae from environmental samples remains difficult. This, together with the lack of standardisation of methods, complicates the interpretation of culture results and comparisons of results from different laboratories. Variations in bacterial numbers in different areas within a water distribution system and the sampling method used often complicate the interpretation of culture results even further.
Previous studies have shown that the culture of Legionella species from environmental samples is complicated by the presence of [13][14] faster growing bacteria due to inhibition of legionellae on culture media in the presence of heterotrophic bacteria. For example,[15] Pseudomonas aeroginosa secrete bacterial substances into the surrounding media that dramatically inhibit Legionella growth. Although culture is still the gold standard, it remains time consuming and requires a certain level of technical skill. Legionellae may also enter a “viable but non-culturable (VBNC)” state under certain conditions which complicated culturing even further.)
| Lp | Lm | L boz | L dum | L gor | L long | L jor | L oak | |
| Growth on BCYE
Growth on TSB Acid production Gelatin hydrolysis Urease Primary growth on FG Beta lactamase Hippurate hydrolysis Browning of tyrosine medium Blue fluorescence on CYE |
+
- - + - + + + + - |
+
- - + - - - - - - |
+
- - + - - +/- - + + |
+
- - + - - + - + + |
+
- - + - - + - + + |
+
- - + - - + or – - + - |
+
- - + - - + - + - |
+
- - - - nt +/- - + - |
| Nt: not tested, +/- weak positiv; L p L. pneumophila; Lm L. micdadei; Lboz L. bozemanii; Ldum L. dumoffii; Lgor L. gormanii; Llong L. longbeacheae; L jor L jordanis; Loak L. oakridgensis. | ||||||||
Recent developments in the molecular field opened doors for new detection assays of waterborne pathogens such as Legionella. These methods include:
- DNA probe hybridisation;[16]
- Restriction enzyme digestion;[17]
- Polymerase chain reaction;[18][19]
- Soluble protein patterns;[20]
- DNA restriction endonuclease profiles;[21]
- Multilocus enzyme analysis;[22]
- Orthogonal-field-alteration gel electrophoresis;[23]
- Sodium dodecyl sulphate poly-acrylamide gel electrophoresis (SDS-PAGE).[24][25]
Polymerase chain reaction (PCR)
Although the sensitivity of most of these techniques is insufficient for direct detection of legionellae in environmental samples, [18][19] PCR has proven to be a sensitive and rapid alternative to culture. Many PCR assays have been described, but relatively few of them have been extensively studied on clinical as well as environmental samples and none are routinely used.
Immunofluorescence
Immunofluorescence is a technique whereby antigen and antibody is bound to a fluorochrome (fluorescent stain) and then allowed to react with the corresponding antigen or antibody on a microscope slide. The results are viewed under a fluorescent microscope. There are many variations of immunofluorescent techniques but only direct immunofluorescence (DFA) and indirect immunofluorescence (IFA) is of importance in the confirmation of environmental legionellae. Direct immunofluorescence (DFA) is most commonly used for confirmation of Legionella species from environmental samples. The test is simple to perform, but interpretation requires a fair amount of experience, especially in highly contaminated samples. Antigen from the sample is fixed to a microscope slide using heat or acetone and covered with fluorescein-isothiocyanate (FITC) labelled globulin. Antigens in the sample bind to the labelled globulin and the resulting antigen-antibody complexes are visible under ultraviolet light. Direct immunofluorescence (DFA) is useful to detect antigens in clinical samples when cultures cannot be obtained, but its value for environmental samples is controversial. Nevertheless, it is used as a screening test by some laboratories. Cross-reactions that may lead to false positive results have been documented.
Fluorescent in situ hybridization
Fluorescent in situ hybridization (FISH) is a technique whereby a fluorescent labeled DNA probe is used to detect a particular chromosome or gene that can then be visualised by fluorescence microscopy. FISH tests are useful for the detection of legionellae in respiratory tract samples but has not been extensively tested in environmental samples. The method makes use of oligonucleotide probes targeting rRNA and offers a fast and specific alternative to direct immunofluorsecence, culture and urine antigen testing in clinical laboratories.
References
- ↑ Adeleke AA, Fields BS, Benson RF, Daneschvar MI, Pruckler JM, Ratcliff RM, Harrison TG, Weyant RS, Birtles RJ, Raoult D and Halablab MA (2001). Legionella drozanskii sp. nov., Legionella rowbothamii sp. nov. and Legionella fallonii sp. nov., three unusual Legionella species. Int. J. Sys. Evol. Microbiol. 51: 1151-1160
- ↑ De Gheldre Y, Maes N, Presti FL, Etienne J and Struelens M (2001). Rapid identification of clinically relevant Legionella species by analysis of transfer DNA intergenic spacer length polymorphism. J. Clin. Microbiol. 39: 162-169.
- ↑ Adeleke AA (1996). Legionella-like amoebal pathogens phylogenetic status and possible role in respiratory disease. Emer. Infect. Dis. 2: 225-230.
- ↑ McCoy WF (2004). Legionella. In: Cloete TE, Rose J, Nel LH and Ford T (eds). Microbial waterborne pathogens. IWA Publishing, UK. ISBN 1 84339 055 8. Pp 100-131
- ↑ Rowbotham TJ (1980). Preliminary report on the pathogenicity of Legionella pneumophila for freshwater and soil amoebae. J. Clin. Pathol. 33: 1179-1183
- ↑ McNealy T, Newsome A, Johnson R and Berk S (2000). Impact of amoebae, bacteria and tetrahymenae on Legionella pneumophila multiplication and distribution in an aquatic environment. In: Marre R, Abu Kwaik Y, Bartlett C, Cianciotto NP, Fields BS, Frosch M, Hacker
- ↑ Kilvington S and Price J (1990). Survival of Legionella pneumophila within Acanthamoeba polyphaga cysts following chlorine exposure. J. Appl. Bacteriol. 68: 519-525
- ↑ Maartens G, Lewis SJ, de Goveia C, Bartie C, Roditi D and Klugman KP (1994). “Atypical” bacteria are a common cause of community acquired pneumonia in hospitalised adults. South African Med. J. 84: 678-682
- ↑ 9.0 9.1 Mauff AC and Koornhof HJ (1984). Legionellosis in South Africa. In: Thornsberry C, Balows A, Feeley JC and Jakubowski W (eds). Proceedings of the 2nd International Symposium on Legionella. American Society for Microbiology, Washington DC
- ↑ Bartie C and Klugman KP (1997). Exposures to Legionella pneumophila and Chlamydia pneumoniae in South African mine workers. Int. J. Occup. Environ. Health 3: 120-127
- ↑ Ratshikhopha ME, Klugman KP and Koornhof HJ (1990). An evaluation of two indirect fluorescent antibody tests for the diagnosis of Legionnaires' disease in South Africa. South African Med. J. 77: 392-395
- ↑ Grabouw NA, Pienaar EJ and Kfir R (1991). The occurrence of Legionella bacteria in cooling towers in South Africa. Wat. Sci. Tech. 24: 149152
- ↑ Bartie C, Venter SN and Nel LH (2001). Identification methods for Legionella from environmental samples. Water Res. 37: 1362-1370
- ↑ Bartie C, Venter SN and Nel LH (2003). Chapter 56. Legionella detection from South African cooling water systems. In: Marre R, Abu Kwaik Y, Bartlett C, Cianciotto NP, Fields BS, Frosch M, Hacker J and Lück PC (eds). Legionella. ASM Press, Washington DC. Pp 284-290. ISBN 1-55581-230-9
- ↑ Hussong D, Colwell RR, O'Brien M, Weiss E, Pearson AD, Reiner RM and Burge WD (1987). Viable Legionella pneumophila not detectable by culture on agar media. Biotechnology 5: 947-950
- ↑ Palmer CJ, Tsai YL, Paszko-Kolva C, Mayer C and Sangermano LR (1993). Detection of Legionella species in sewage and ocean water by the polymerase chain reaction, direct fluorescent staining and plate culture methods. Appl. Environ. Microbiol. 59: 3618-3624
- ↑ Bej AK, Mahbubani MH DiCesare JL and Atlas RM (1991). Polymerase chain reaction gene probe detection of microorganisms by using filter-concentrated samples. Appl. Environ. Microbiol. 57: 3529-3534
- ↑ 18.0 18.1 Mahbubani MH, Bej AK, Miller R, Haff L, DiCesare J and Atlas RM (1990). Detection of Legionella pneumophila with polymerase chain reaction and gene probe methods. Mol. Cell. Probes 4: 175-187
- ↑ 19.0 19.1 Ferguson DA Jr. and Mayberry WR (1987). Differentiation of Legionella species by soluble protein patterns in polyacrylamide slab gels. Microbios. 52: 105-114.
- ↑ Lo Presti F, Riffard S, Meugnier H, Reyrolle M, Lasne Y, Grimont PA, Grimont F, Benson RF, Brenner DJ, Steigerwalt AG, Etienne J and Freney J (1998). Legionella gresiliensis sp. nov. and Legionella beliardensis sp. nov. isolated from water in France. J. Clin. Microbiol. 36: 193-197
- ↑ Woods TC, McKinney RM, Plikaytis BD, Steigerwalt AG, Bibb WF and Brenner DJ (1988). Multilocus enzyme analysis of Legionella dumoffii. J. Clin. Microbiol. 26: 799-803
- ↑ Ehret W, Anding G, Tartakovski I and Ruckdeschel G (1993). Molecular epidemiology of outbreak-associated Serogroup 1 isolates. In: Barbaree JM, Breiman RF and Dufour AP (eds). Legionella: Current status and emerging perspectives. American Society for Microbiology, Washington DC. Pp 223-225
- ↑ Diogo A, Veríssimo A, Nobre MF and Da Costa MS (1999). Usefulness of fatty acid composition for differentiation of Legionella species. J. Clin. Microbiol. 37: 2248-2254
- ↑ Ng DLK, Koh BB and Heng BH (1997). Comparison of polymerase chain reaction and conventional culture for the detection of legionellae in cooling tower waters in Singapore. Lett. Appl. Microbiol. 24: 214
- ↑ De Klerck P, Vereist L, Duvivier L, Van Damme A and Olivier F (2003). A detection method for Legionella spp in (cooling) water: fluorescent in situ hybridisation (FISH) on whole bacteria. Wat. Sc. Tech. 47: 143-146
THE CHAIN OF INFECTION
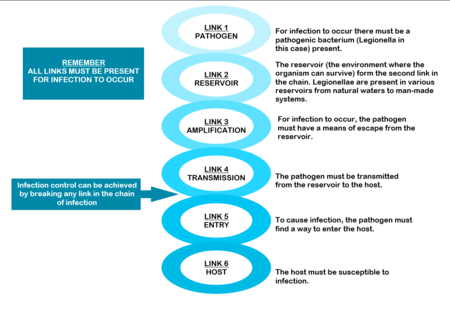
The mere presence of legionellae in a water distribution system does not necessarily imply a human health risk. For human infection to occur, certain conditions are necessary. These conditions are referred to as the “chain of infection” consisting of six links. All the links have to be present for disease to occur (Diagram: Chain of infection ). The first link, the pathogen, was discussed in Section 1.
SOURCES AND RESERVOIRS
Legionellae are natural inhabitants of water, found a wide range of habitats. They are ubiquitous in streams, lakes and rivers. They also survive in dust, soil and mud. In fact, one of the species, Legionella longbeacheae, is so often isolated from potting soil in Australia that soil has been suggested as the natural habitat of this particular species.

Legionellae from these natural environments can be transmitted to man-made water systems by various means. For example, from raw water, during water treatment, as part of post-treatment after-growths within water distribution systems, during building and construction activities and during plumbing repair.
Once established, they can persist in the water supply for long periods of time and are difficult to eradicate. Therefore, their presence must be considered in all aspects of the design, operation and maintenance of buildings. For this to be effective, cooperation between engineers, occupational health practitioners and microbiologists is essential.
Figure 2.3 Man-made sources of Legionella
Water sources that provide optimal conditions for Legionella growth can be separated into those containing “non-potable” and those that contain “potable” water. Non-potable water distribution systems
Heat rejection devices like cooling towers, evaporative condensers and HVAC (heating, ventilation and air-conditioning) systems are often implicated as sources of legionellosis. They contain reservoirs filled with warm, recirculating water that makes them ideal for the growth, amplification and dissemination of micro organisms (including Legionella). In a typical water-cooled system air in induced through or blown over, packing material down which water, circulating from a pond under the packing, is allowed to fall by gravity, producing a large wetted surface that cools the falling water.
The constant fall of water through the tower, the large area of the basin, fill, pipes and heat exchanger, the warm temperature of the water, the high relative humidity and high organic content within these devices provide conditions that favour contamination by algae, protozoa, fungi and bacteria. The risk is increased further by the open nature of the systems, excessive aeration and the constant addition of fresh water to make up for water lost through evaporation.
In systems that are not regularly cleaned, sludge accumulates in the reservoir and slime adheres to water covered surfaces, resulting in the presence of large concentrations of micro-organisms, including legionellae, on these surfaces. In addition, water temperatures below 60°C, the age and configuration of the system, the pH of the water and the presence of certain metals may also increase the risk of contamination.
Water derived from municipal supplies but subsequently stored in cisterns, or conditioned prior to heating, is considered non-potable due to the deterioration in chemical and bacteriological quality during storage. Colonisation of such non-potable sources inside large buildings, such as hotels, factories or hospitals, may be a major cause of legionellosis.
Potable (domestic) water distribution systems
Legionellae are often present in potable water supplies, especially in the hot water sections of these systems. The organisms may enter potable water supplies from the main source, even from municipal water, and survive standard treatment protocols because most municipal water systems are not routinely screened for the presence of legionellae and the organisms are chlorine tolerant. Once inside the system, they find a suitable environment to multiply and are usually very difficult to eradicate.
Legionella levels can rise from very low to very high within short periods of time. The factors that give rise to these fluctuations are not well understood and often very hard to determine. These factors include the age and configuration of the pipes, the degree of scaling and sediment and the potential for biofilm formation within the system increase the risk of contamination. Water temperatures of 25 – 42°C, stagnation and the presence of certain free-living amoebae capable of supporting the intracellular growth of legionellae are often mentioned as amplifying factors in published reports. The biofilm and scale that form on surfaces in water distribution systems provide nutrients for legionellae and protect them from hot water and disinfectants. Some materials used in these systems, for example neoprene washers, are more readily colonised than others (See Table). Building location may also play a role in the colonisation of potable water with legionellae.
Hot water tanks are often colonised with legionellae, especially at the bottom where a warm zone may develop and scale and sediment accumulate. Hot water piping, especially dead-legs, presents an additional risk as legionellae thrive in stagnant water.
Soil
Outdoors, the soil may be contaminated through contact with Legionella-polluted water and become a source of airborne bacteria during earth moving operations, such as construction work.
|
Very good |
Copper |
|
Good |
Other synthetic materials |
|
Reasonable |
Steel |
|
Not recommended |
Rubber Plastics |
Amplification
Legionellae are usually present in low numbers in natural sources. However, certain factors present in man-made reservoirs can promote Legionella growth and amplification. To improve our understanding of Legionella, its potential to cause disease and how to better control the organisms in water systems, we must understand these conditions. The most important factors amplifying Legionella numbers in man-made reservoirs are listed in the table Amplifying factors for Legionella in man-made sources and reservoirs.
Remember Temperature data is usually based on laboratory studies and is not from actual system (piping) studies, which makes it even less reliable to use for Legionella control. System temperature on its own should therefore not be relied upon for Legionella control, because the so-called “system temperature” rarely indicates one uniform temperature throughout the entire system. Therefore, maintaining the system temperature does not guarantee Legionella control. Also, in plumbing systems, especially larger and/or more complex piping systems, legionellae have been shown to survive at even higher temperatures due to biofilm, dead-legs, and other complexities. It has been suggested that potable water systems be operated at temperatures as high as possible but take into account the risk of scalding injuries and energy conservation requirements.
|
TEMPERATURE |
|
|
pH |
|
|
STAGNATION |
|
|
WATER TREATMENT |
|
|
DISINFECTANTS |
|
|
CHEMICAL PARAMETERS |
|
|
RELATIVE HUMIDITY |
|
|
SLIME, ALGAE AND PROTOZOA |
|
|
CORROSION PRODUCTS |
|
|
CONSTRUCTION |
It is believed that legionellae are released from the soil during excavations from where they can enter the cooling tower of the building, air intakes or water pipes, or may be inhaled directly. Another possibility is that, during construction, nutrients already present in dust and dirt may become more readily available for the organisms. In new buildings, plumbing should be flushed before use. Renovated buildings may contain stagnant water, that should be flushed out before returning the building to normal use.
|
|
WATER PRESSURE |
|
|
BIOFILM, SCALE AND SEDIMENT |
· Legionellae have been found in biofilms forming on plastic surfaces in water piping systems. At a temperature of 40° they were shown to account for approximately 50% of the total biofilm flora; · Legionellae are less likely to be present on copper surfaces because copper generally do not support biofouling. If present, the bacteria are usually found in small numbers; · Metal plumbing components and associated corrosion products provide iron and other metals needed for Legionella, thereby supporting their survival and growth.
Algal slime also provides a stable habitat for their survival and multiplication.
|
Disinfectants After disinfection, municipal water supplies usually travel several kilometers before it reaches the point of use. During this course, disinfectant residuals diminish and there is increasing exposure to potentially biofilm-contaminated piping. Although municipal water systems are required to be disinfected at their points of distribution to conform to existing standards for bacterial disinfection, these standards are based upon the absence of coliform bacteria and do not include any specific testing requirements for Legionella.
Transmission
After growth and amplification of legionellae to potentially infectious levels, the next requirement in the chain of infection is to achieve transmission of the bacteria to a susceptible host. Modern technology like cooling towers used to recirculate water for air-conditioning and humidifying purposes and other ventilation systems can cause the formation and distribution of aerosols through which the organisms can spread. Transmission can also occur through direct installation, aspiration or ingestion (Table Dissemination of Legionella bacteria).
|
AEROSOLISATION |
|
|
DIRECT INSTALLATION |
|
|
INGESTION |
|
|
ASPIRATION |
|
INFECTION AND HOST SUSCEPTIBILITY
Infections caused by Legionella species are collectively known as legionellosis and include Legionnaires’ disease and Pontiac fever. Subclinical (asymptomatic) infections have been reported. Legionellosis occurs worldwide, in people of all ages and race groups, with no evidence of person-to-person spread of infection. It is most common in summer and autumn months. The incidence of legionellosis varies from country to country and from region to region. Recently, an increase in the worldwide incidence of reported legionellosis cases has become evident. This may be explained by the availability of improved diagnostic and testing methods, increased awareness of the symptoms and improved surveillance. However legionellosis, especially sporadic cases, is still not always reported to public health authorities, making it difficult to estimate its true incidence.
The mode of transmission, inoculum size, particle size and host susceptibility influence the severity of infection. Approximately half of the currently known Legionella species are implicated in disease, but pneumophila is still considered to be the causative agent in about 80% of diagnosed cases. However, this picture might change as the number of available diagnostic tests increases – it is thus important to regard all legionellae a potentially pathogenic until proven otherwise.
Section 3
LEGIONELLA INFECTIONS
Infections caused by Legionella species are collectively known as legionellosis and include Legionnaires’ disease and Pontiac fever.1,2 Subclinical infections have been reported. Legionella infections occur worldwide in people of all ages and race groups with no evidence of person-to-person spread of infection.3,4
LEGIONNAIRES’ DISEASE
Legionnaires’ disease (LD) is a severe multisystem disease with pneumonia as the most predominant clinical finding. Clinical features are similar to those of other pneumonias, making it difficult to diagnose.5,6 Symptoms range from a mild cough and slight fever to a coma with widespread pulmonary infiltrates and multisystem failure. Survivors usually recover completely although lung fibrosis and neurological abnormalities may persist in some cases. LD has a low attack rate but the mortality rate is high.
Legionnaires’ disease outbreaks occur frequently all over the world. In the United States, Legionnaires’ disease is considered to be fairly common and legionellae are among the top three causes of sporadic, community-acquired pneumonia. However, many cases are still not reported, as Legionnaires’ disease is difficult to distinguish from other forms of pneumonia. Although only approximately 1,000 cases are reported to the Centers for Disease Control and Prevention (CDC), it is estimated that over 25,000 cases occur every year, causing more than 4,000 deaths.
Despite this, only one outbreak has been reported in South Africa to date. However, previous South African studies indicated antibody levels to L pneumophila in 65% of healthy blood donors, 36% of healthy mineworkers, 10% of healthy factory workers and 16% of hospitalised pneumonia patients.7,8 One study reported seroconverion to L.
pneumophila in 9% of patients hospitalised between 1987 and 1988 with symptoms of community-acquired pneumonia.9
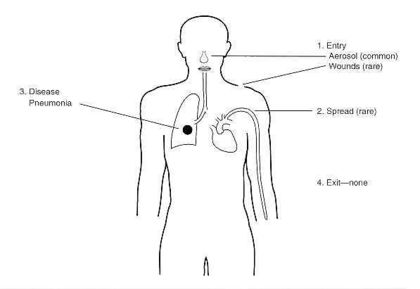
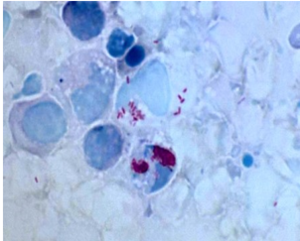
Figure 3.1 Legionella pathology
RISK FACTORS
In order for LD to occur, the host must be susceptible to infection. Older people (above 50 years of age) are more commonly infected. Men are more likely to be infected (ratio 3:1) but the racial distribution is usually consistent with that of the population involved.10
Table 3.1 lists the most common risk factors.
| Table 3.1 Risk factors for development of Legionnaires’ disease | |
|---|---|
| Patient demographics | Smoking
Chronic pulmonary disease Immunosuppression Renal transplantation Renal dialysis Alcohol ingestion Age > 50 years Male |
| Environmental risks | Exposure to construction activities
Exposure to air conditioning systems Exposure to home air conditioning Travelling and accommodation in hotels Potable water Hospitalization |
Source: Winn 1984 10
SYMPTOMS
Legionnaires’ disease presents with a broad spectrum of symptoms, ranging from mild cough and low fever to stupor, respiratory and multi organ failure. Pneumonia is the predominant clinical finding. Early symptoms are mainly non-specific and include fever, malaise, myalgias, anorexia and headache.6,11 The temperature often exceeds 40°C and the patient may present with a slightly productive cough. Chest pain, occasionally pleuritic, can be prominent and when coupled with hemoptysis, may mistakenly suggest pulmonary emboli. Gastrointestinal symptoms (watery stools) are prominent, especially diarrhoea, which occurs in 20-40% of cases. Relative bradycardia has been over-emphasised as a diagnostic finding but is often seen in elderly patients with advanced pneumonia. Hyponatremia (serum sodium concentration ≥ 130 mmol/l) occurs more frequently in Legionnaires’ disease than in other pneumonias.
Extrapulmonary Legionnaires’ disease is rare but the clinical manifestations are often dramatic. These infections can easily be overlooked since the degree of suspicion is generally low in these cases. Legionella has been implicated in cases of sinusitis, cellulitis, pancreatitis, peritonitis and pyelonephritis. The most common extrapulmonary site of infection is the heart. There have been numerous reports of myocarditis, pericarditis, postcardiotomy syndrome and prosthetic valve endocarditis. In most cases there is no pneumonia symptoms present. Wound infections have also been reported.11
INCUBATION PERIOD
LD has an incubation period (the time it takes for symptoms to appear after exposure) of 2 – 10 days. The onset of symptoms may be sudden or gradual.
DIAGNOSIS
There are no reliable distinguishing clinical features to distinguish LD from pneumonia caused by other etiologic agents. However, there are some clinical clues to assist in the diagnosis (Table 3.2).6
| Table 3.2 Clinical clues to Legionnaires’ disease | |
|---|---|
| CLUE | EXAMPLE |
| PATIENT HISTORY AND PHYSICAL EXAMINATION | |
| Presence of an epidemic or documented source of infection | Family, friends or associates with similar infection and exposure |
| Prominent neurologic or gastrointestinal symptoms | Pneumonia with confusion, nausea and vomiting |
| Non-response to aminoglycosides or beta-lactam antibiotics | Worsening condition after 5 days on antibiotics |
| LABORATORY RESULTS OF PATIENT | |
| Gram stain of sputum with many neutrophils but no bacteria | Laboratory reports showing many neutrophils and few normal flora or no bacteria |
| Nodular peripheral infiltrates in chest radiographs | Progression of unilateral opacities to bilateral nodular infiltrates over several days |
It is important to remember that a clinical diagnosis of LD always has to be confirmed with specialised laboratory tests. As not all laboratories are equipped to perform these tests routinely, the tests have to be specifically requested by the physician. Table 3.3 highlights some of the most commonly used laboratory tests.12
- Culture of Legionella organisms from clinical samples is still the gold standard for diagnosing LD. The technique is highly specific, provided appropirate samples are used, and about 1.5 to 3 times more sensitive than immunofluorescence. Transtracheal aspirates are best for culture, but sputum, bronchial aspirates, pleural exudates, lung biopsies as well as wound swabs and even autopsy material have been used successfully.11 Disadvantages of the culturing of legionellae for diagnostic purposes include possible inhibition by non-legionellae organisms present in the sample, slow growth and difficulties in distinguishing legionellae from other organisms on solid media. These factors must be taken into account when choosing a laboratory to test clinical samples for LD.
- Immunofluorescence is useful to detect antigens (direct immunofluorescence) or antibodies (indirect immunofluorescence) in clinical samples in cases where culture is not possible. Cross reactions with organisms other than Legionella in the direct immunofluorescence (DFA) test may cause false positive results, making accurate interpretation of the results essential.11,13 Indirect immunofluorescence (IFA) is the most specific of the currently available serological tests for LD. It is reproducible, sensitive and specific for the diagnosis of especially L. pneumophila infections, but may be affected by several factors, including the method of antigen preparation, method of antigen fixation during preparation of the reagent, the class of immunoglobulin it is designed to detect and strain differences.14,15
- The Legionella Urinary Antigen test5 is a relatively inexpensive and rapid diagnostic test, but only detects infections caused by L pneumophila Serogroup 1. The test is commercially available as a radioimmunoassay (RIA) or an enzyme linked immunosorbent assay (ELISA). An advantage of this test is the relative ease with which urine samples can be obtained, especially in patients with a non-productive cough. Legionella antigens may persist in the urine of some patients for as long as one year.
- Serological tests are useful for epidemiologic studies but less valuable for physicians. Diagnosis by serology is based on a fourfold rise in antibody titre to ≥ 1:128 in paired samples (from the acute and convalescent stage of disease).13,15 However, the antibody response may not be detectable until 1-3 months after onset of illness. Single titres of ≥1:256 during convalescence in pneumonia patients is suggestive of legionellosis. Antibody screening should include both IgG and IgM because some patients may only have an IgM response.5
- Assays based on the polymerase chain reaction (PCR) have been used to detect legionellae in urine, broncho-alveolar lavage fluid and sputum. These tests are highly specific but not more sensitive than culture and are much more expensive to perform. Limitations of PCR tests include the possible presence of certain “PCR inhibitors” in sputum and blood samples. The major advantage of PCR is the rapidity of the test and the ability to detect species other than L pneumophila. PCR is not used routinely for the clinical diagnosis of LD.
| Table 3.3 Sensitivity and specificity of laboratory tests for the diagnosis of Legionnaires’ disease | ||
|---|---|---|
| TEST | SENSITIVITY (%) | SPECIFICITY (%) |
| Culture from clinical samples | 80 | 100 |
| Direct immunofluorescence (DFA) | 33-70 | 96-99 |
| Indirect immunofluorescence (IFA) | 40-60 | 96-99 |
| Urinary antigen detection | 70 | 100 |
Source: Stout and Yu, 1997
WHAT TO TAKE INTO ACCOUNT WHEN INTERPRETING LABORATORY RESULTS
- Both IgM and IgG should be measured simultaneously.
- Diagnostic IgM titres will provide an earlier diagnosis than IgG because they indicate a primary immune response.
- Results obtained by the IFA should always be interpreted in conjunction with the clinical presentation of the disease.
- Titres below the diagnostic level together with clinical manifestations may be useful for early provisional diagnosis of Legionnaires’ disease; but diagnosis by IFA is usually retrospective.
- The interpretation of the IFA should take into account the variation in the time of appearance of antibodies, the types of antibodies produced and the length of time the antibodies are detectable in sporadic cases, as well as the prevalence of antibodies in the population from which the patient comes.
- The type of reagents used for IFA tests may influence the results: ether-killed, formalin-killed or heat-killed antigens vary in sensitivity and specificity and this should be taken into account in the interpretation.
- False negative results may be reported because a long time is needed for seroconversion to occur and not all species and serogroups are detectable by this method.
- Seroconversion (a fourfold rise in titre to at least 1:128) is considered as a presumptive positive result.
- A single titre of 1:256 or higher is regarded as a presumptive positive result.
- Serological results should preferably be confirmed by culture.
- In communities with low antibody prevalence, a single titre of 1:128 may be diagnostic and where the prevalence is high, a single titre of 1:256 may still provide only presumptive evidence of infection.
- Low titres usually indicate past infections.
- When titres to multiple antigens are raised, the titre that is fourfold higher than the others is considered to be diagnostic.
- In epidemiological studies diagnostic titres are usually one twofold dilution higher than for sporadic cases.
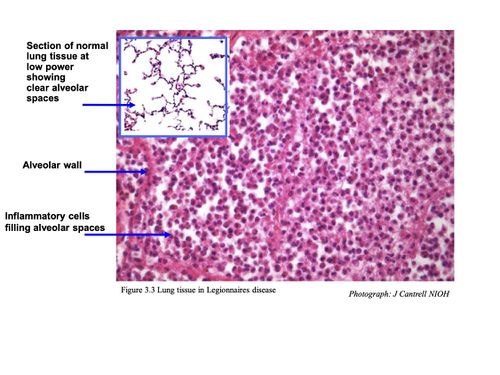
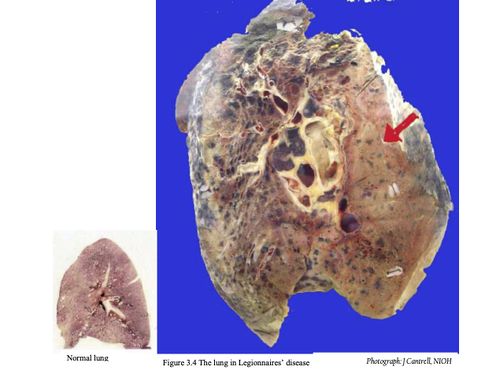
HISTOLOGY
Pulmonary lesions usually consist of a mixture of neutrophils and macrophages, fibrin, proteinaceous material and red blood cells. Neutrophils and macrophages are often present in the centre of a lesion with mainly macrophages around the periphery.
Intracellular bacteria are present in both neutrophils and macrophages. Further away from the site of acute inflammation, bacteria are mainly seen inside the macrophages.10
CHEST RADIOGRAPHS
Radiographic features in Legionnaires’ disease are mostly non-specific, and absent in Pontiac fever. Abnormalities occur from the third day post infection in most Legionnaires’ disease patients and usually do not correlate well with the severity of illness.11 However, the abnormalities correlate with the presence of the Legionella bacterium in sputum. The time required to show clearing of infiltrates on radiographs is variable and may range from 1-4 months. Some patients show diffuse alveolar damage. In the majority of patients with Legionnaires’ disease:
- Initial involvement is unilateral, predominantly in the lower lobe
- Bilateral involvement has been described
- Initial densities are poorly marginated, homogenous, rounded, occur either on the periphery or in the centre of the lung and may be mistaken for pulmonary infarction
- Pulmonary densities enlarge during later stages of disease
- Pulmonary densities have a typical ground glass appearance or dense consolidation
- Total opacification of the lung may occur
- Pleural effusions are present in 24-63% of cases caused by L pneumophila
- Pleural effusions are uncommon in L micdadei infections
- Hilar adenopathy seldom occurs
- Cavitation may occur in immunocompromised patients
- Cavitation rarely occurs in L micdadei infections
Figure 3.4 Chest radiograph of Legionnaires’ disease patient
TREATMENT
Treatment of LD requires the use of antibiotics. However, many antibiotics effective against other bacterial pneumonias are ineffective against Legionella as these drugs do not penetrate the pulmonary cells (alveolar macrophages) where infectious Legionella bacteria thrive.
Erythromycin was historically the drug of choice for the treatment of Legionnaires’ disease, but the newer macrolides (azithromycin) and quinolones (ciprofloxacin, levofloxacin, moxifloxacin, gemifloxacin, trovofloxacin have superior in vitro activity and greater intracellular and lung-tissue penetration.12 Other agents that have been shown to be effective include tetracycline, doxycycline, minocycline, trimethoprim- sulfamethoxazole.12 Rifampin is recommended as part of combination therapy with a macrolide or a quinolone for patients who are severely ill. The total duration of therapy is usually 10-14 days; however a 21-day course may be needed for immuno-compromised patients or those with extensive evidence of disease on chest radiographs.12
When LD patients are treated with appropriate antibiotics near the onset of disease, the prognosis is usually very good, especially if there is no underlying illness compromising the immune system. For patients with compromised immune systems, including transplant patients, any delay of appropriate treatment may result in complications, prolonged hospitalisation and death. However after successful treatment and hospital discharge, many patients still experience fatigue, loss of energy and difficulty concentrating. These symptoms may persist for several months, but complete recovery usually occurs within one year.
PONTIAC FEVER
Pontiac fever is an acute, self-limiting, flu-like illness without symptoms of pneumonia. The first outbreak of Pontiac fever was reported in Pontiac, Michigan, in 1968.16
It is characterised by high fever, chills, myalgia and malaise but without the pneumonia or cough typical of Legionnaires’ disease. Some authors suggest that it is a hypersensitivity pneumonitis, caused either by infection with a free-living amoeba called Acanthamoeba filled with legionellae or as a result of a toxic reaction to the organism. The incubation period is short, ranging from 1 – 3 days, and the attack rate high, exceeding 90% in some cases. The fatality rate is low.
Pontiac fever symptoms usually resolve spontaneously within one week, only symptomatic treatment is needed and the chest radiograph is clear. There is no evidence of secondary spread of the infection in Pontiac fever. Diagnosis can only be made by seroconversion, which may be delayed for up to 6 weeks after onset of symptoms. Cases of PF have been linked to L pneumophila, L feelei and L anisa. Complete recovery usually occurs in 2 – 5 days without medical attention.
CHAPTER 4
SURVEILLANCE FOR LEGIONELLA
A major aspect of biosafety in the workplace is the assessment of hazards and risks of infection (environmental surveillance) and disease in workers (clinical surveillance). ENVIRONMENTALSURVEILLANCE Environmental surveillance is essential for the long term success of any water treatment program for Legionella to provide baseline environmental information to ensure the efficiency of disinfection and water treatment programmes and to control the risk of infection in events of mechanical failures and human error. The risk of contracting legionellosis can be summarised in terms of three factors, as summarised in Table 4.1. Table 4.1 Factors determining the risk of legionellosis Proliferation potential This potential exist in nearly all water systems and depends on many factors, including: Presence of microbial biofilms Diversity of microorganisms present Availability of nutrients Exposure potential Certain industrial processes produce aerosols during their function. These aerosols can be aspirated (breathed deep into the lung) if the particle size is small enough (< 8 µm in diameter). Assessment of exposure potential thus focuses on the proximity of aerosol-generating systems to human populations. Population susceptibility The most susceptible individuals are elderly people (especially males), heavy smokers, alcoholics, patients with chronic pulmonary disease and immuno-suppressed people. 1 Source: McCoy 2004 4.1 RISK ASSESSMENTS A risk assessment is a scientifically based process used to estimate the likelihood of adverse effects occurring, taking into account the exposure (hazard), the level and nature of the risk and the target population. Risk assessments can be either quantitative or 1 qualitative. 4.1.1 Quantitative risk assessments Quantitative risk assessments involve the establishment of dose-effect and dose-response relationships in likely target individuals and populations. For a quantitative risk assessment to be successful, dose-response assessments, the extent and duration of human exposure and characterisation of the possible consequences resulting from exposure have to be determined 1 and quantified. Quantitative risk assessments are not possible for hazardous biological agents (including Legionella) for several reasons. Firstly, exposed people have varying degrees of susceptibility to infection. Secondly, water sources usually contain a diverse mixture of biological contaminants which may change rapidly over short periods of time. Thirdly, the contaminants present and the levels in which they are present are only applicable at the time and area sampled and the interactions among all these 1 biological agents present in the source and environment make exposure assessment extremely difficult. 4.1.2 Qualitative risk assessments Qualitative risk assessments on are done to “document site-specific conditions and recommendations for reducing the risk, if 1 necessary, and to establish assignments for actions, communications, training and management responsibilities”. Anumber of schemes have been developed to provide some form of risk ranking and risk scores to simplify these assessments 1 and to guide the water management plan. An example of a risk ranking for legionellosis is provided in Table 4.2. For example, if a system falls into Risk Category A, the frequency of testing, cleaning and disinfection and monitoring should be increased until the system falls into a lower Risk Category, when the rate of testing, cleaning and monitoring can be reduced again. 19 Table 4.2 Risk classification for legionellosis HIGHEST RISK LOWEST RISK RISK CLASSIFICATION A B C D CRITERIA MATCHES ANY OF THE RESPONSES BELOW MATCHES ANY OF THE RESPONSES BELOW AND NONE IN A MATCHES ANY OF THE RESPONSES BELOW AND NONE IN A OR B MATCHES ANY OF THE RESPONSES BELOW AND NONE IN A, B OR C Stagnant water System idle more than once a month Re-circulation pump not timed Dead-legs present A plus timed recirculation pump Any single factor in A System operates continuously No dead-legs Nutrients and growth Contaminated water No corrosion control Wet surfaces open to sunlight Any two factors in A Any one factor in A No factors in A Water quality No automated biocide dosing protocol No water treatment program No automated biocide dosing No water treatment program in place Automated biocide dosing No water treatment program in place Automated biocide dosing Water treatment program in place Equipment design and operation No system design review No system operation and performance review No drift eliminators Drift eliminators for cooling towers in place Same as B with at least one review lacking System design review in place System operation and performance review in place Good drift eliminators Location and access Located in healthcare or residential care facility Large numbers of people exposed Same as A but moderate numbers of people exposed Same as B but few people exposed Not located near susceptible people 1 Source: McCoy 2004 4.2 THE RISK ASSESSMENTPROCESS The four most important steps in the risk assessment process are to get an overview of the operation of the water distribution system, to conduct a walk-through investigation of the building or facility, to assess the results of the walk-through to determine an 2,3,4 appropriate course of action and to recommend appropriate control actions. These steps are explained in more detail below. STEP 1 GET AN OVERVIEW OF THE WATER DISTRIBUTION SYSTEM OPERATION Ask the facilities engineer or an experienced member of the building staff, who has knowledge of the design and current operation of the system to explain the system and to assist in the walk-through investigation. The overview should include inspection of: ! Plumbing systems, heating/ventilation/air-conditioning (HVAC) systems and other water reservoirs as summarised in Table 4.3 20 ! Maintenance records of all water systems, including records of temperature checks on potable water, details of visual and physical checks of cooling towers and reports of water quality assessments and chemical treatment that may have been performed ! Location of parts of the system where there may be stagnant water ! The presence of cross-connections between potable and non-potable water systems ! The condition and type of back flow prevention devices used at these connections ! Any recent major maintenance actions performed or major changes in the system ! Determine whether recent or frequent losses of water pressure have occurred from the incoming supply ! Keep in mind that the failure of a back flow prevention device under loss of pressure can result in contamination of the water system Table 4.3 Areas to include when doing an overview of a waterdistribution system Plumbing systems Hot and cold potable water systems Water heaters Water coolers Distribution piping Water treatment equipment (eg. water softeners, filters) Connections to process water systems (not protected by backflow preventers) Storage tanks Heating, ventilation and airconditioning (HVAC) systems Cooling towers Evaporative condensers Fluid coolers Direct and indirect evaporative cooling equipment Humidifiers Location of fresh air intakes relative to water sources Air washers for filtration Miscellaneous reservoirs Decorative fountains Plant misters Whirlpools and spas Tepid water eye washes Safety showers Cooling water for industrial processes 2 Source: http://www.nalco.com STEP 2 CONDUCT A WALK-THROUGH INVESTIGATION OF THE FACILITY Table 4.4 The walk-through investigation WHAT TO DO WHEN ! Check sample transport requirements and receiving times of laboratory before investigation ! Get as much information as possible from staff before investigation ! Go through checklist and make sure you are prepared before investigation ! Check water temperatures while sampling for Legionella ! Check hot water tanks and related piping during investigation ! Check cooling towers during investigation ! Check water quality during investigation ! ! ! ! ! WHO SHOULD BE PRESENT? The person who knows most about the building, particularly the domestic water system Aplumbing engineer if possible Aplumbing contractor who knows the building if possible Arepresentative from the company that services the HVAC system The water treatment specialist who services the cooling tower 21 EQUIPMENT NEEDED ! The floor plan (arrange in advance if possible) ! Drawings of plumbing if available (arrange in advance if possible) ! Inspection checklist ! Camera ! Knife ! Pliers or channel locks ! Measuring tape SAMPLING KITS AND EQUIPMENT TO TAKE WITH ! Thermometer ! Chlorine kit ! Iron kit ! pH meter ! Total dissolved solids (TDS) kit ! Hardness kit ! Calibration fluid ITEMS NEEDED FOR LEGIONELLA SAMPLING ! Sampling log (see example) ! Sodium thiosulfate ! Cooler bag and other materials needed for packaging of sample for transport ! Documentation from courier or transport agency ! Plastic bags for packaging of samples ! Sterile swabs ! Sampling bottles ! Labels for bottles ITEMS NEEDED FOR SAMPLING FOR THE TOTAL BACTERIAL COUNT (TBC) ! Sampling bottles ! Sampling log sheets ! Cooler bag and other materials needed for packaging of sample for shipping or transport ! Documentation from courier or transport agency ! ! REMEMBER The temperature may be below the gauge temperature of the heater due to heat stratification CHECKLIST FOR FAUCETS CONNECTED TO EACH WATER HEATER ! Maximum temperature of each location that is “close (C)”, “intermediate (I)” or “far (F)” from the heaters ............................................................................................................ REMEMBER You may have to run the water for several minutes before it reaches a maximum temperature at “far” locations CHECKLIST FOR COLD WATER STORAGE TANKS USED FOR RESERVE WATER OR FOR MAINTAINING HYDROSTATIC PRESSURE ! InitialTemperature (°C) .......................................................................................................................... ! Potential for stagnation: high (H), medium (M) or low (L)........................................................................ ! Temperature of cold water line at various locations Location ............................................................... Temperature (°C) ......................................... Location ............................................................... Temperature (°C) ......................................... Location ............................................................... Temperature (°C) ......................................... Location ............................................................... Temperature (°C) ......................................... Record both initial temperature and final equilibration temperature on cold water lines ...................... REMEMBER Tanks should be protected from temperature extremes and should be covered to prevent stagnation CHECKLIST FOR EACH STORAGE-TYPE HOT WATER HEATER Temperature of water drawn from it (°C) ................................................................................................. Presence of rust and scale ......................................................................................................................... 22 CHECKLIST FOR COOLING TOWERS, EVAPORATIVE CONDENSERS AND FLUID COOLERS ! Visual evidence of biofilm growth, scale build-up and turbidity ........................................................... ! The location of the tower relative to fresh air intakes ............................................................................ ! Proximity to sources such as kitchen exhausts, leaves, plant materials, or other sources of organic material that may contribute to Legionella growth .............................................................................................. CHECKLIST FOR COOLING TOWERS ! General physical and mechanical condition ........................................................................................... ! Presence and condition of drift eliminators ............................................................................................ ! Basin temperature of water (the tower is working) ................................................................................ REMEMBER Wear appropriate respiratory protection (half face-piece respirator and HEPA filter cartridge) during examination if cooling tower is operating SUMPS FOR COOLING TOWER, EVAPORATIVE CONDENSER AND FLUID COOLERS ! Location and condition ............................................................................................................................ ! Location of any cross-connections between potable and non-potable systems ......................................... REMEMBER Sumps are sometimes located indoors to prevent them from freezing. Cross-connections may be present to supply a back-up source of cool water to refrigeration condenser units or to supply secondary cooling units STEP 3 ASSESS THE RESULTS OF THE WALK-THROUGH TO DETERMINE AN APPROPRIATE COURSE OF ACTION Table 4.5 When is additional action necessary? NO FURTHER ACTION Operating temperatures measured in water heaters is 60°C Delivery temperature at distant faucets is ? 50°C No other potential problems observed TAKE FURTHER ACTION Poor maintenance Operating temperatures below minimums mentioned above STEP 4: RECOMMEND CONTROLACTIONS Remember! These actions may include disinfection of the potable water system as outlined in the section on disinfection 23 Table 4.6 Recommended control actions ACTION EXPLANATION Actions to limit the growth of organisms in water systems: Elimination of dead legs in the plumbing system Insulation of plumbing lines Installation of heat tracing to maintain proper system temperatures Elimination of rubber gaskets Removal or frequent cleaning of plumbing fixtures (eg aerators and shower heads) Water sampling to confirm the presence of Legionella: Not necessary at this stage BUT Customer may want to obtain background information, in which case sampling for Legionella will be helpful To ensure that corrective actions are successful: Sample water after corrective action BUT Customer may want to collect samples before corrective action to assess the extent of the problem but this is not normally done The customer should take the necessary corrective action, even if the pre-sampling tests are negative for the following reasons: Water sampling may produce false-negative results A contaminated portion of the system may have been missed The absence of legionellae at the time of sampling does NOT ensure that the system will remain negative at a later date Limited corrective actions that will not be sufficient: Raising water heater temperature without evaluation of system points of stagnation, heat loss and heat gain, cross-contamination and other factors that may contribute to the growth of Legionella bacteria. LEGIONELLA RISK ASSESSMENT DATA SHEET GENERAL INFORMATION Name of organisation Contact person 1 Contact person 2 Contact person 3 Site address Mailing address Building/area size Building age Type of building 24 RESULTS FROM PREVIOUS LEGIONELLA TESTING DATE RESULT Cooling towers Hot water tanks Outlet swabs Hot water outlets Cold water outlets Mixed outlets Incoming water 25 USEFUL QUESTIONS TO ASK ABOUT THE FACILITY WHEN DOING A RISK ASSESSMENT FOR LEGIONELLA(SOURCE: FREIJE 2001) Incoming mains Number Configuration Water source City - ground City - surface Utility: Private well Water usage Obtained from Water bills Fixture count Meter reading Other Deadlegs Present Policies Past construction Aerators Yes No Type Cold pipes location relative to hot Above Below Same level Pipe material Pipe insulation Cold Hot Both Type used Drinking fountains On chilled loop Individual 26 Fire sprinkler above ducts Yes No Water hammers arrestors or air chambers Water hammers Air chambers Both Sediment on water filters Yes No Other sediment gathering equipment Yes No Describe Connection control and/or backflow devices Yes No Faucets and showerheads Age Condition Water softened Cold water Yes No How Hot water Yes No How Water pressure shock Incidents Brown colour Building units closed off temporarily Yes No Major construction activities Yes No Dates Eye wash stations Mixed Cold Emergency showers Mixed Cold 27 Ice machines present Yes No Decorative fountains present Yes No Piped in coffee/tea/cooldrink centres present Yes No Rubber hoses present Yes No Misters for plants/food/comfort present Yes No Other equipment where water flow through that may produce a spray/mist Yes No Can windows be opened? Yes No HVAC systems +Condition of coils Overall condition Drainage of drip pans Humidifiers in duct? Hot water system Number of HW loops Number of CW loops HW recirculating? CW recirculating? Design temp at tank Design temp at taps Regulators Mixing valves Sensors to detect failures CW storage tanks present? Are they covered? Heaters or sunlight? 28 HOT WATER SYSTEM INSPECTION Model number Serial number Age Horizontal/vertical Condition Insulation Temp on gauge Temp from drain Temp of returning water Capacity/turnover Cleaning regimen Cleaning frequency All pumps used daily? Piping arrangement Draw diagram on separate sheet and attach 29 COOLING TOWER INSPECTION In-house identification Location Make and model Serial number Size Age Basin drainable? Cycling of each Months operating Water treatment company Contact details Cleaning frequency Who does the cleaning How is cleaning done? Is basin fully drained? Excessive foaming? Oily film on surfaces? Scum? Condition of drift eliminators Air bypassing: Around edges? Through holes in tower casing? Fill exposed to sunlight Basin exposed to sunlight Louvres/fill clogged? Louvres/fill damaged? Louvres/fill scale present? Louvres/fill algae present? Filters SAND BAG CARTRIDGE Pressure drop 30 Centrifugal separators Outlet strainers Pressure drop Corrosion/deterioration on Structural members? Safety rails? Ladders? Fasteners? Basin? Water leaks present? Air leaks present? Sump water level Cooling water temp Accessible to passers by? Distance from road Distance from parking Distance from sidewalks 31 COOLING TOWER INSPECTION CHECKLIST 5 Source: Freije 2001 BUILDING TOWER NAME/NUMBER INSPECTOR’S NAME DATE OF INSPECTION SAND FILTERS Water leaks? YES NO Suction air leaks? YES NO Pre-strainer clogged? YES NO Channeling in sand filter media (check quarterly) YES NO Operating pressure If YES, clean the media or replace it with filter sand designed specifically for cooling towers and evaporative condensers (not swimming pools). Clean the under-drain assembly while the media is removed. CENTRIFUGAL SEPARATORS Pressure drop Acceptable based on manufacturer’s chart? YES NO Flow rate Purge operation functional? YES NO UNUSUAL NOISE OR VIBRATION IN Pumps? YES NO Motors? YES NO Fans? YES NO Mounting hardware? YES NO OUTLET STRAINERS In place? YES NO Clogged? YES NO BAG AND CARTRIDGE FILTERS Pressure drop Acceptable based on manufacturer’s recommendations? YES NO If not, clean or replace TOWER STRUCTURE AND CASING Water leaks? YES NO Air leaks? YES NO 32 CORROSION OR OTHER DETERIORATION ON Structural members? YES NO Safety rails? YES NO Ladders? YES NO Fasteners? YES NO Basin? YES NO LOUVERS, FILL AND DRIFT ELIMINATORS Clogged? YES NO Damaged? YES NO Excessive scale or algae growth? YES NO If yes, clean with 6,895 – 10,343 kPa (1,000 – 1,500 psi) water, being careful not to damage fill and eliminator components WATER DISTRIBUTION Is the water distribution balanced? YES NO If not, unclog nozzles SEDIMENT BUILD-UP Sediment build-up around heater elements? YES NO FANS Do fans start and stop properly? YES NO VIBRATION Are the vibration switches operating properly? (check at least annually) YES NO SEASONAL OPERATION Are the seasonal systems working (check at least 3 months before winter) YES NO Are there any parts needed? YES NO If YES, what is needed? Are there any repairs necessary? YES NO If YES, what is needed? 2133 Describe action taken in response to above inspection (include dates): QUESTIONS FOR INFECTION CONTROL COORDINATOR (IF APPLICABLE) History of cases Cleaning of hydrotherapy baths and pools Practices for use and cleaning of portable humidifiers Practices for use and cleaning of nebulizers and other semi-critical respiratory equipment Water treatment for dialysis Contact details: Dental unit present? How is it treated? Contact details: Clinical tests for Legionella available? Policy for testing patients/workers for Legionella? 34 CHECKLIST FOR LEGIONELLA SAMPLING HW tank PRE-FLUSH POST-FLUSH Incoming water Faucets and showerheads Cooling make-up water Decorative fountains Cooling tower basins WATER QUALITY TESTS SAMPLE LOCATION pH Free chlorine Total chlorine Hardness TDS Iron TBC Coliforms WATER TEMPERATURES SAMPLE LOCATION HW after 1 minute (°C) CW after 1 minute (°C) 35 EXAMPLE OFSAMPLING LOG / REQUESTFORM REQUESTFOR TESTING OFWATER SAMPLES FOR LEGIONELLA COMPANYDETAILS .................................................................................................................................................................................................. CONTACTPERSON .................................................................................................................................................................................................. CONTACTDETAILS TEL........................................... FAX ............................................. E-MAIL................................................................ NAME OF PERSON WHO COLLECTED SAMPLES ............................................................................................................................... COLLECTION SITE .................................................................................................................................................................................................. SAMPLE NO LOCATION TYPE TIME COLLECTED COMMENTS Received by ……………………………..……….... Date ……………………….. Time ……………… HEALTH SURVEILLANCE Health surveillance can be defined as the “ongoing collection, comparison, analysis and dissemination of data relevant to public health issues”. The main objectives of health surveillance are to identify disease patterns in different population groups, to develop prevention strategies, to allocate resources for prevention and treatment and to educate health professionals and the general public. Health surveillance can take the form of: ! The notifiable disease reporting system (the first step in the surveillance cycle) ! Laboratory based surveillance (for diseases that can be monitored accurately through the laboratory data used for confirmation of the disease) 36 ! Hospital-based surveillance (where hospital discharge information and mortality data are used to monitor trends and disease burden in a particular area) ! Population-based surveillance (where data from a well-defined population are collected and analysed) DISEASE NOTIFICATION Disease notification serves as a means of collecting data on diseases that are considered to be of public health importance and is used world wide. Most countries (including South Africa) have a routine notification system requiring health professionals to report the occurrence of cases and deaths related to certain notifiable conditions. In South Africa, 39 medical conditions are currently 6 notifiable (Table 4.7). The notification system in South Africa is based on Health Act No. 63 of 1977 together with certain Regulations on the reporting of specific diseases to the Local, Provincial and National Health Departments. Table 4.7 Notifiable conditions in South Africa Acute flaccid paralysis Leprosy TB (pulmonary) Any person (not necessarily a health care worker) may report a notifiable condition to a local authority via fax, mail or telephone. The relevant notification form is then completed by the local authority or district office, and submitted to the provincial office, which in turn summarises the cases and deaths for each notifiable condition (even if no cases or deaths occurred during that period) and sends the summary to the national office. WHO IS RESPONSIBLE FOR NOTIFICATION? The first health care professional or facility with whom a patient presenting with one of the notifiable medical conditions comes into contact is expected to notify the case (or death). This includes clinic personnel, infection control nurses, other hospital staff, and public and private medical practitioners. In cases where the patient had no contact with a health care professional, a member of the community is required to report the case. WHYIS NOTIFICATION SO IMPORTANT? Healthcare professionals have a legal obligation to report cases of notifiable conditions. The process of notification alerts outbreak response teams, resulting in appropriate and timely interventions, in turn assisting in the prevention of spread of communicable diseases. In addition, the notification system provides information on the status and trends of communicable diseases in the country. THE NOTIFICATION PROCESS There are certain forms to be filled in when reporting a case or death. These are available from the regional health office, the local Communicable Disease Coordinator or the provincial stores section. ! Form GW17/05: Initial diagnosis (to be completed immediately and containing finer details of the patient and the diagnosis) ! Form GW17/03: Line list of cases (to be completed weekly) ! Form GW17/04: Line list of deaths (to be completed weekly) Notifications are done using GW17/05 forms. The structure of the system, process of obtaining the forms and persons to which the forms are sent differ considerably between, and even within provinces. People at the provincial health information offices, however, are conversant with specific details of the system in their province, and nurses and doctors are encouraged to contact them for specific details about how to go about notifying cases and deaths of the diseases above. Alist of telephone numbers and addresses 7 of these contact people is included at the end of this article. Asemi-detailed overview of the process is described below. The GW17/05 forms are required to be completed as fully as possible, although information on patient's age, sex or race may be omitted only if it is not available. All other applicable information should be filled in, especially details relating to the place and date of infection, the disease being notified and whether a case or death is being notified. The contact people in the relevant provincial health information office will be able to furnish you with the details of the specific office to which the notification forms should be 37 sent. The steps to follow when reporting notifiable conditions are summarised in Table 4.8. Table 4.8 Steps in the notification process STEP 1 Diagnose and confirm that the patient is suffering/has died of a notifiable condition. STEP 2 Obtain the necessary documentation: Form GW 17/5 In the case of death the condition should be reported twice, first as a case and then as a death. STEP 3 Complete all the forms and send them to the relevant office: Local Authority/Hospital/District responsible for disease containment (fill in Form GW 17/3); Regional Office (the Health Information Unit); Provincial Office (the Health Information Unit); National Department (Directorate HSR and Epidemiology) 7 Source: The notification system in a nutshell. Remember! When workers complain of symptoms, the first question to be answered is not "How do we fix the building?", but rather: “Why to they have symptoms?". In other words, to address health complaints effectively, a health evaluation is absolutely essential. A systematic approach to legionellosis in the work environment is crucial.
Acknowledgements
Delene Bartie (CB Scientific)- 2023
References Chapter 3
- Dowling JN, McDaevitt DA, Pasculle AW. Isolation and preliminary characterization of erythromycin-resistant variants of Legionella micdadei and Legionella pneumophila. Agents Chemother. 1985, 27 (2): 272-274.
- MacFarlane JT, Miller AC, Smith WH, Morris AH, Rose DH. Comparative radiographic features of community acquired Legionnaires’ disease, pneumococcal pneumonia, Mycoplasma pneumonia and psittacosis. Thorax 1984, 39: 28-33.
- Boldur I, Beer S, Kazak R, Kahana H, Kannai Y. Predisposition of the asthmatic child to legionellosis? Isr. J. Med. Sci 1986, 22 (10): 733-736.
- Kurtz JB. Legionella pneumophila. Am. J. Occup. Hyg. 1988, 32 (1): 59-61.
- Shapiro M. Unusual epidemiologic and clinical manifestations of legionellosis: a review. Isr. J. Med. Sci. 1986, 22 (10): 724-727.
- Yu VL. Legionella pneumophila (Legionnaires’ disease). In: Mandell, Douglas and Bennet (eds). Principles and practice of infectious disease. 1990, Third Edition, Churchill Livingstone.
- Ratshikhopha ME, Klugman KP, Koornhof HJ. An evaluation of two indirect fluorescent antibody tests for the diagnosis of Legionnaires’ disease in South Africa. South African Med. J. 1990, 77: 392-395.
- Bartie C and Klugman KP. Exposures to Legionella pneumophila and Chlamydia pneumoniae in South African mine workers. Int. J. Occup. Environ. Health 1997, 3: 120-127.
- Maartens G, Lewis SJ, de Goveia C, Bartie C, Roditi D, Klugman KP. “Atypical” bacteria are a common cause of community acquired pneumonia in hospitalised adults. South African Med. J. 1994, 84: 678-682.
- Winn 1991
- Roig J, Aguilar X, Ruiz J, Domingo C, Mesalles E, Manterola J, Morera J. Comparative study of Legionella pneumophila and other nosocomial-acquired pneumonias. CHEST 1991, 99: 344-350.
- Stout JE, Yu VL. Legionellosis. New Engl. J. Med. 1997, 682-687.
- Ruf B, Schürman D, Horbach I, Fehrenbach FJ, Pohle HD. Prevalence and diagnosis of Legionella pneumonia: a 3-year prospective study with emphasis on application of urinary antigen detection. J. Inf. Dis. 1990, 162: 1341-1348.
- Harrison TG, Dournon E, Taylor AG. Evaluation of sensitivity of two serological tests for diagnosing pneumonia caused by Legionella pneumophila serogroup 1. J. Clin. Pathol. 1987, 40: 77-82.
- Pastoris MC, Ciarrochi S, Di Capula A, Temperanza AM. Comparison of phenol- and heat-killed antigens in the indirect immunofluorescence test for serodiagnosis of Legionella pneumophila serogroup 1 antigens. J. Clin. Microbiol. 1984, 20 (4): 780-783.
- Kaufmann AF, McDade JE, Patton CM, Bennett JV, Skalyi P, Feeley C, Anderson DC, Potter ME, Newhouse VF, Gregg MB, Brachman PS. Pontiac fever: isolation of the etiologic agent (Legionella pneumophila) and demonstration of its mode of transmission. Am. J. Epid. 1981, 114 (3): 337-347.
- Dennis PJ (1993). Potable water systems: insights into control. In: Barbaree JM, Breiman RF and Dufour AP (eds). Legionella: Current status and emerging perspectives. American Society for Microbiology, Washington DC. Pp 223-225.
- Colbourne JS and Dennis PJ (1985). Distribution and persistence of Legionella in water systems. Microbiol. Sc. 2: 40-43.
- Muraca PW, Stout JE, Yu VL and Yee YC (1988). Mode of transmission of Legionella pneumophila. A critical review. Am. J. Hyg. Assoc. 49: 584-590.
- Yamamoto H, Sugiura M, Kusunoki S, Ezaki T, Ikedo M and Yabuuchi E (1992). Factors stimulating propagation of legionellae in cooling tower water. Appl. Environ. Microbiol. 58: 1394-1397
- Jump up to:5.0 5.1 Freije MR (1996). Legionella control in healthcare facilities: A guide for minimising risk. HC Information Resources, Inc. United States of America. P 8
- MarrieTJ, Haldane D, Bezanson G and Peppard R (1992). Each water outlet is a unique ecological niche for Legionella pneumophila. Epid. Infect. 108: 264-270
- ↑ Cite error: Invalid
<ref>tag; no text was provided for refs named:0