Infection Prevention and Control/Air Disinfection
Implementation of Upper Room UVGI
Introduction and context
This guide is a compilation of current best practice and knowledge regarding the design, development and operation of indoor room air ultraviolet germicidal irradiation (UVGI) systems for reducing the rate of transmission of airborne diseases such as tuberculosis (TB).
This guidance document is not intended for the applications of water or surface disinfection.
Effectiveness of UV
The disinfection effectivity of UVGI and the susceptibility of airborne microorganisms including M. tuberculosis (TB) bacilli (peak sensitivity at around 265nm) have been scientifically proven.
Safety
UV-c has a lower skin penetration depth, thus does not easily cause skin irritation or cancers when compared to UV-a and UV-b found in sunlight. UV-c does cause eye irritation at high exposure levels.
Therefore, UVGI in occupied rooms should not exceed an exposure dose of 6 mJ/cm² (for mercury vapour lamps at 254 nm 15) and 3.8 mJ/cm² (at 265nm) per 8h. The potential of high UV intensities being reflected from certain materials (e.g. reflectors of regular open luminaires, windows, exposed ducting and metallic or high gloss architectural finishes) into the occupied portion of the room must be considered by designers and users.
During any work in the upper room or with open UVGI devices, eye and skin protection should be worn.
Applications and definitions
The application of UVGI should not be seen as a substitute for, rather a component of, a comprehensive IPC policy.
Upper room UVGI
These devices irradiate air and inactivate pathogens in the unoccupied zone of the upper room. During normal air circulation, the air exchange between the upper and lower room reduces the concentration of viable airborne pathogens in the whole room.
Air cleaners
Room air cleaners consisting of enclosed UVGI lamps in housings, circulate air to achieve a measure of clean air delivery to the room. The advantages are related to safety aspects and lower maintenance costs.
Whole room UVGI
Whole room UVGI disinfection attempts to sterilise room surfaces by exposing the entire room volume to high UV fluence levels. This method is ineffective for reducing airborne transmission within occupied rooms.
Planning and procurement
The uncontrolled use of UVGI without a comprehensive management policy can result in fruitless expenditure and create a false sense of protection, thereby potentially exposing building occupants to increased risk. The management policy should align with the regional and national IPC policies and should include sourcing and procurement, maintenance, training, decommissioning and disposal of UVGI devices.
Design and installation
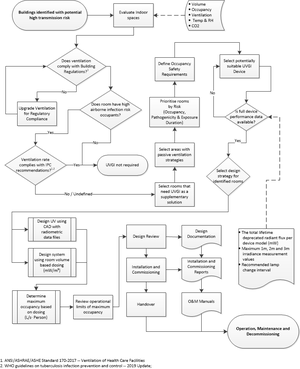
Design lifecycle
The UVGI design life cycle should include system planning, space evaluation, design and review, control of the design and commissioning documents. Risk prioritization and reverting to more conventional infection control strategies should be considered. Interference from obstructions (light fixtures, beams, ductwork, piping, furniture, equipment or non-prescribed lighting diffusers) to airflow around the device or radiation from the device must be taken into account.
Two design processes are briefly described.
Radiometric design process
Two rational design methods are available.
The first recommended rational design process follows the guidance of CIE 155:200315 and the design completion will need quantification and verification of:
- Selected UVGI device’s radiometric data
- Considered pathogen’s UV-c sensitivity (Z-value)
- Considered pathogen’s infectious dose
- Air exchange rates and ventilation efficiency parameters
The design objective is a prescribed reduction in transmission of 80% or higher.
The second recommended rational design process is available through the application of design software targeting prescribed whole-room radiant flux levels. However, this method is only appropriate for open type UVGI devices.
Prescriptive design process
The prescriptive design process aims to determine the required number of UVGI devices for the considered indoor space and the resultant occupancy limits in accordance with the WHO recommended minimum ventilation rate of 80 l/s per person. This design process is applicable for well-mixed room air, achieved through the installation of mixing fans. If the considered room has an existing and functional mechanical ventilation system, the outside air portion of that system’s ventilation rate should be included in the calculation to increase the occupancy limit determination. The design process assumes that UV-reflective surfaces do not affect eye safety.
The following set of device and application data is required:
- Total maintained UVGI radiant flux <math display="inline">\bigl(\Phi_T , mW \bigr)</math> of selected devices, measured in accordance with SATS 1706:2016 as amended and the UVGI measurement and instrumentation and calibration plan
- The selected device’s minimum equivalent clean air delivery rate <math display="inline">\bigl(CARD_e , L/s \bigr)</math>.
- Room volume <math display="inline">\bigl(V_r , m^3 \bigr)</math>, occupancy profile <math display="inline">\bigl(N_p , number of persons \bigr)</math> and effective mechanical ventilation rate (Qv, l/s) of considered room.
- <math>Number of devices_\phi=\frac{14\times V_r}{\phi_T}</math>
- <math>CADR_e=\frac{\bigl( 80\times N_p \bigr) -Q_v}{Number of devices_{CADR} }</math>
It is strongly recommended that the room be labelled with clear and informative signage to indicate the TB related occupancy limits with the UV system turned on and turned off.
Acceptance criteria
Design requirements for effectiveness can be assessed through compliance with the following:
- Minimum required UV volumetric fluence rate (MRU) of 14mW/m³ or
- A UVGI equivalent ventilation rate of the product of 80 l/s per person and the typical peak number of room occupants. This CADRe rate determines the total required UV-c output.
Any shortfall between the required and the actual ventilation rate or MRU should be corrected for increasing by the number of UVGI devices installed, as determined using the formulae in section § Prescriptive Design Process. The number of devices required should not be less than the minimum number determined, or exceed this value by more than 1.
Alternatively, for rooms with unknown occupancy levels, the CADRe resulting from the number of devices determined by the prescribed MRU could be used to define the safe occupancy limit for that room The UV dose should inactivate airborne infectious agents by at least 90% (D90) or a percentage equivalent of the ventilation rate of 80 l/s/per person as recommended by the WHO.
Lamp Ageing
A 100-hour lamp burn-in is recommended prior to the device characterisation or system verification tests. The lamps of dimmable systems should be burnt-in at full output for the initial seasoning period.
Factors affecting performance
UVGI disinfection performance is dependent on good air movement rates either through natural convection or mechanically aided (e.g. paddle fan). The effectiveness may be reduced if the mechanical ventilation rates in a room are increased together with equivalent room air mixing. However, as ventilation is a primary environmental control against indoor airborne transmission, its maximum capacity within the considered area should be determined and targeted before implementing a UVGI solution.
Installation
The device must not be able to tilt or swing under normal operation.
The minimum installation height of the lower horizontal plane of any open UVGI device above the finished floor level is determined from the table below.
| Ceiling Height (m) | Recommended Mounting Height (m): | ||
|---|---|---|---|
| Corner type (90°) | Wall type (180°) | Pendant type (360°) | |
| 2.4 | 2.2 | 2.2 | 2.4 |
| 2.7 | 2.3 | 2.3 | 2.4 |
| 3.0 | 2.4 | 2.4 | 2.4 |
In the typical application of 254 nm LPMV devices, occupant eye exposure shall not exceed and average of <math>0.10 \mu W\cdot cm^-2</math> for a period of time equivalent to 8 hours per day. The strategy for factoring equivalent acceptable exposure levels in the lower room in typical healthcare settings is represented in the table below.
| ZONE | EYE LEVEL (m) |
ESTIMATED DAILY EXPOSURE TIME (h) |
IRRADIANCE LIMIT (uW/cm²) |
|---|---|---|---|
| Corridors (no waiting) | 1.7 | 1 | 0.8 |
| Indoor Waiting Areas | 1.7
1.2 |
3 | 0.3 |
| ICU | 1.7 1.0 |
4 6 |
0.2 0.15 |
| Bedrooms/Offices | 1.7 1.2 |
2 4 |
0.4 0.2 |
| Wards/Dormitories | 1.7 1.2 |
2 4 |
0.4 0.2 |
| Isolation Rooms | 1.7 1.2 |
2 4 |
0.4 0.2 |
| Consultation Rooms | 1.7 | 2 | 0.4 |
| Bronchoscopy rooms | 1.7 | 2 | 0.4 |
| Autopsy Rooms | 1.7 | 2 | 0.4 |
| Dining Halls | 1.7 | 2 | 0.4 |
| Dormitories | 2.1 | 8 | 0.1 |
| Other areas | 1.7 | 4 | 0.2 |
Commissioning and start-up
The system start-up should commence after the installation process is complete. Prior to start-up and the initial verification testing, lamps must be cleaned and seasoned according to the manufacturer’s instructions. Device performance should be verified independently, by switching all devices off then measuring each device’s output individually. The output must concur with the initial specification stipulated in the procurement bid. The 1m and 2m maximum irradiance (measured at 1m and 2m from the external front face of the device) for each device must comply with the manufacturer’s declared performance values. Using declared lifetime low performance values, each new or refurbished device will be independently accepted for service when it is proven to exceed the declared minimum or depreciated performance values by at least 43% (100/70).
Example:
<math>65 mW.cm^{-2} </math> -(declared 2m irradiance)
<math>65\times1.43=92.95 mW.cm^{-2} </math> -(acceptance measurement at 2m)
Eye safety measurements should only be conducted after performance verification tests are successfully completed.
Documentation
The following reports and records are required upon handover:
- User requirement specification
- Design report
- Performance verification report
- Operation and maintenance manual
The operation and maintenance manual (O&M) should include the following information:
- Designer’s trade name, contact and professional or technical registration details.
- Room selection methodology; alternatively, where this selection was not made by the design consultant, a copy of the design brief.
- Electrical wiring drawings and compliance certificate covering all building electrical work relating to the UVGI installation.
- Plan and elevation drawings of considered rooms indicating device installation details, supporting structures and obstructions to radiation and airflow.
- Design data, including assumptions such as room occupancy and ventilation levels.
- Selected device performance criteria and datasheets.
- Selected lamp data sheets including rate lamp life and details of 3 alternate lamps and suppliers.
- Startup and shut down procedures.
- Maintenance and monitoring protocol, plan and log-book template.
- Initial performance and safety verification acceptance criteria.
- Performance verification reports
- Device cleaning instructions.
- Spare parts list with recommended stock levels.
- Decommissioning and disposal instructions
Monitoring and maintenance
In every workplace which may result in persons being exposed to hazardous biological agents (HBA) in the performance of their duties, the R1390 Government Gazette of 27 December 2001 No. 22956 is applicable. This legislation requires that the employer shall ensure that all control measures for transmission-based precautions are maintained in good working order; and that thorough examinations and tests of engineering control measures - such as UVGI - are carried out at intervals not exceeding 24 months by an approved inspection authority or their verified delegate. Record of the examinations and tests carried out in terms of regulation and of any repairs resulting from these investigations and tests must be kept for at least three years. If the maintenance and monitoring is done in-house it should be conducted by trained and competent personnel. A maintenance plan shall be developed for each installation and include cleaning and component replacement as per manufacturer’s instructions. It is recommended that maintenance tags be put on all devices. A predictive maintenance model is recommended to determine frequency of maintenance interventions. System level maintenance and monitoring plans can initially be derived from generic templates but should be reviewed and updated regularly.
Irradiance measurements should be taken in the same location and orientation for repeatability between measurements. These locations can be physically marked in the room.
Corrective actions
If individual devices fail initial performance measurement upon lamp re-cleaning and ballast replacement, then the devices should be identified clearly with a red non-conformance sticker and scheduled for decommissioning and disposal. Any new ballasts and lamps installed in failed devices should be immediately salvaged for re-use.
If the number of unserviceable devices within a considered space and system results in an insufficient number of functional devices; the entire system serving that space shall be declared as non-compliant. Individual devices declared functional within a non-functional system may be salvaged for re-use.

Training
Competence and training of both system owners and service providers are critical to the successful and sustainable implementation of UVGI air disinfection systems. Professional service providers can be trained either by professional institutions or through tertiary or post graduate education. System owners can be trained by the service provider or can outsource training from a reputable and accredited training institution. An example of a responsibility matrix including the minimum training topics to be covered is presented in the complete guidance document.
Acknowledgements
Edward A. Nardell (Brigham and Women's’ Hospital), Richard L. Vincent (Mount Sinai Medical Center), Steve N. Rudnick (Harvard School of Public Health), Paul Jensen (US Centers for Disease Control), Lindiwe Mvusi (NDoH), FW Leuschner (University of Pretoria). The development of the guidance document was supported by the Presidents Emergency Plan for Aids Relief (PEPFAR) grant together with the US-CDC. The content of this report does not necessarily reflect the opinion of the funders or those acknowledged or referenced in its development.
Key References
- IUVA Draft Guideline IUVA-G02A-2005 International Ultraviolet Association Guideline for Design and Installation of UVGI Air Disinfection Systems in New Building Construction Copyright 2005 International Ultraviolet Association
- Fundamental Factors Affecting Upper-Room Ultraviolet Germicidal Irradiation—Part II. Predicting Effectiveness
- World Health Organisation. WHO Policy on TB Infection Control in Health-Care Facilities, Congregate Settings and Households. Geneva, Switzerland; 2004.
- World Health Organisation. Infection Prevention and Control of Epidemic- and Pandemic-Prone Acute Respiratory Diseases in Health Care. Geneva, Switzerland, Switzerland; 2007.
- World Health Organisation. Natural Ventilation for Infection Control in Health-Care Settings. Geneva, Switzerland; 2009. http://www.who.int/entity/water_sanitation_health/publications/natural_ventilation.pdf. Accessed April 30, 2013.
- Downes A, Blunt T. Researches on the effect of light upon bacteria and other organisms. Proc R Soc London. 1877;26:179-184. http://rspl.royalsocietypublishing.org/content/26/179-184/488.full.pdf. Accessed May 28, 2014.
- Gates FL. A study of the bactericidal action of ultra violet light: III. The absorption of ultra violet light by bacteria. J Gen Physiol. 1930;14:31-42. http://jgp.rupress.org/content/14/1/31.full.pdf.
- Riley R. Guidelines for the Application of Upper-Room Ultraviolet Germicidal Irradiation for Preventing Transmission of Airborne Contagion — Part I : Basic Principles. 1999.
- Wells WF, Fair MG. Viability of B. coli exposed to ultra-violet radiation in air. Science (80- ). 1935;82:280-281.
- Riley RL, Permutt S. Room air disinfection by ultraviolet irradiation of upper air. Arch Environ Heal An Int J. 1971;22(2):208-219. http://www.tandfonline.com/doi/pdf/10.1080/00039896.1971.10665834#.U4Ym1fmSxyU.
- Riley RL, Permutt S. Convection, air mixing, and ultraviolet air disinfection in rooms. Arch Environ Heal An Int Journal1. 1971;22:200-207.
- Riley RL, Permutt S, Kaufman JE. Room air disinfection by ultraviolet irradiation of upper air: further analysis of convective air exchange. Arch Environ Heal An Int J. 1971;23(2):35-39.
- Escombe AR, Moore DAJ, Gilman RH, et al. Upper-room ultraviolet light and negative air ionization to prevent tuberculosis transmission. Wilson P, ed. PLoS Med. 2009;6(3):e43. doi:10.1371/journal.pmed.1000043.
- Nardell EA, Bucher SJ, Brickner PW, et al. Safety of upper-room ultraviolet germicidal air disinfection for room occupants: results from the Tuberculosis Ultraviolet Shelter Study. Public Health Rep. 2008;123(1):52-60. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2099326&tool=pmcentrez&rendertype=abstract.
- Nardell EA. Environmental control of tuberculosis. Med Clin North Am. 1993;77(6):1315-1334. http://europepmc.org/abstract/MED/8231415. Accessed June 9, 2014.
- NIOSH US Department of Health, Education and Welfare PHS. Criteria for a Recommended Standard Occupational Exposure to Ultraviolet Radiation.; 1972. doi:(HSM) 73-110009.
- CIE Technical Division 6. CIE 155: 2003 Ultraviolet Air Disinfection. Vienna, Austria; 2003. doi:ISBN 978 3 901906 25 1.
- The International Commission on Non-Ionizing Radiation Protection. Guidelines on Limits of Exposure to Ultraviolet Radiation of Wavelengths between 180 Nm and 400 Nm (Incoherent Optical Radiation). Vol 87.; 2004. doi:10.1097/00004032-200408000-00006.
- R.L. Riley, M K, G M. Ultraviolet susceptibility of BCG and virulent tubercle bacilli. Am Rev Respir Dis. 1976;113:413-18.
- Mundt E, Nielsen P V. Ventilation Effectiveness.; 2004.
- SATS 1706: 2015 SOUTH AFRICAN TECHNICAL STANDARD UVGI Luminaires - Safety and performance requirements.
- Beggs CB, Noakes CJ, Sleigh P a., Fletcher L a., Kerr KG. Methodology for determining the susceptibility of airborne microorganisms to irradiation by an upper-room UVGI system. J Aerosol Sci. 2006;37(7):885-902. doi:10.1016/j.jaerosci.2005.08.002.
- Illuminating Engineering Society of North America (IESNA) Testing Procedures Committee. Standard File Format for Electronic Transfer of Photometric Data and Related Information (ANSI/IESNA LM-63-02). New York; 2002.
- F.W. Leuschner. UVGI MEASUREMENTS, INSTRUMENTATION & FACILITIES PLAN. Pretoria; 2014.
- Zhang J, Levin R, Angelo R, et al. A radiometry protocol for UVGI fixtures using a moving-mirror type gonioradiometer. J Occup Environ Hyg. 2012;9(3):140-148. doi:10.1080/15459624.2011.648569.
- Illuminating Engineering Society of North America (IESNA) Testing Procedures Committee. IES Guide for Lamp Seasoning (LM-54-91). New York; 1991.
- First MW, Banahan KF, Dumyahn TS. Performance of ultraviolet germicidal irradiation and luminaries in long-term service. Leukos. 2007;3:181-188. doi:10.1582/LEUKOS.2007.03.03.005.
- South African Bureau of Standards. SANS 50285 : 2010 SOUTH AFRICAN NATIONAL STANDARD Energy Efficiency of Electric Lamps for Household Use Measurement Methods.; 2010.
- Vorlander FJ, Raddin EH. The effect of operating cycles on flourescent lamp performance. Illum Eng. 1950.
- Miller S.L., Hernandez M., Fennelly K, et al. Environmental Control for Tuberculosis: Basic Upper-Room Ultraviolet Germicidal Irradiation Guidelines for Healthcare Settings. Cincinnati, OH; 2009. http://www.cdc.gov/niosh/nas/rdrp/appendices/chapter6/a6-23.pdf.
- Xu P, Peccia J, Fabian P, et al. Efficacy of ultraviolet germicidal irradiation of upper-room air in inactivating airborne bacterial spores and mycobacteria in full-scale studies. Atmos Environ. 2003;37(3):405-419. doi:10.1016/S1352-2310(02)00825-7.
- Ko G, First MW, Burge H a. Influence of relative humidity on particle size and UV sensitivity of Serratia marcescens and Mycobacterium bovis BCG aerosols. Tuber Lung Dis. 2000;80(4-5):217-228. doi:10.1054/tuld.2000.0249.
- Miller S, Hernandez M, Fennelly K. Efficacy of ultraviolet irradiation in controlling the spread of tuberculosis. Atlanta Centers Dis …. 2002. http://scholar.google.com/scholar?hl=en&btnG=Search&q=intitle:Efficacy+of+ultraviolet+irradiation+in+controlling+the+spread+of+tuberculosis#0. Accessed August 15, 2014.
- Mphahlele M, Dharmadhikari AS, Jensen PA, et al. Institutional Tuberculosis Transmission: Controlled Trial of Upper Room Ultraviolet Air Disinfection - A Basis for New dosing Guidelines. Am J Respir Crit Care Med. 2015:150504122726005. doi:10.1164/rccm.201501-0060OC.
- Noakes CJ, Beggs CB, Sleigh PA, Kerr KG. Effect of Room Mixing and Ventilation Strategy on the Performance of Upper Room Ultraviolet Germicidal Irradiation Systems. In: Proceedings of ASHRAE IAQ 2004. ; 2004:1-13.
- First M, Rudnick SN, Banahan KF, Vincent RL, Brickner PW. Fundamental factors affecting upper-room ultraviolet germicidal irradiation - part I. Experimental. J Occup Environ Hyg. 2007;4(5):321-331. doi:10.1080/15459620701271693.
- ASHRAE. ASHRAE 55-2013; Thermal environmental conditions for human occupancy. Am Soc Heating, Refrig Air Cond. 2013.
- Coker I, Nardell E, Fourie B. Guidelines for the Utilisation of Ultraviolet Germicidal Irradiation (UVGI) Technology in Controlling the Transmission of Tuberculosis in Health Care Facilities in South.; 2001. http://www.sahealthinfo.com/tb/guidelines.pdf. Accessed June 30, 2014.
- Kowalski W, Bahnfleth W. Proposed standards and guidelines for UVGI air disinfection. IUVA News. 2004;6(1):20-25. http://www.aerobiologicalengineering.com/stds.pdf.
- Rudnick SN, First MW. Fundamental factors affecting upper-room ultraviolet germicidal irradiation - part II. Predicting effectiveness. J Occup Environ Hyg. 2007;4(5):352-362. doi:10.1080/15459620701298167