Difference between revisions of "Infrastructure Guidance for COVID-19/Alternate Care Sites"
(→CSSD) |
|||
| Line 594: | Line 594: | ||
==== Functional requirements ==== | ==== Functional requirements ==== | ||
The most basic equipment needed in a laundry includes washing machines, tumble dryers and ironing machines. Equipment requiring steam is not recommended for a temporary facility. The sizing of the laundry, equipment and engineering services can be modified based on the principles provided in the IUSS Laundry and linen. | The most basic equipment needed in a laundry includes washing machines, tumble dryers and ironing machines. Equipment requiring steam is not recommended for a temporary facility. The sizing of the laundry, equipment and engineering services can be modified based on the principles provided in the IUSS Laundry and linen. | ||
| − | + | === Catering services === | |
Kitchenettes, that is, areas for tea, coffee and snacks, mainly for staff, in staff pause areas are discussed elsewhere in this document. | Kitchenettes, that is, areas for tea, coffee and snacks, mainly for staff, in staff pause areas are discussed elsewhere in this document. | ||
Catering services (for staff and patients) may be provided on- or off-site. If the ACS is to be established with easy access to a suitable, existing, functional kitchen service (e.g. hotel, military or hospital catering) which can meet the additional demand of the ACS, then this should be used. If there is no suitable facility, catering should be outsourced via a suitable off-site supplier. Only in the event that no feasible or suitable, existing facility or local supplier is available, should a new catering service be established at the ACS. Detailed guidance for the sizing, design and layout of catering services can be found in the IUSS Catering Services for Hospitals. | Catering services (for staff and patients) may be provided on- or off-site. If the ACS is to be established with easy access to a suitable, existing, functional kitchen service (e.g. hotel, military or hospital catering) which can meet the additional demand of the ACS, then this should be used. If there is no suitable facility, catering should be outsourced via a suitable off-site supplier. Only in the event that no feasible or suitable, existing facility or local supplier is available, should a new catering service be established at the ACS. Detailed guidance for the sizing, design and layout of catering services can be found in the IUSS Catering Services for Hospitals. | ||
| Line 600: | Line 600: | ||
It is recommended that patient and staff meals, where provided, be supplied in disposable, containers, suitable for incineration, and that these are disposed of as risk waste immediately after use. | It is recommended that patient and staff meals, where provided, be supplied in disposable, containers, suitable for incineration, and that these are disposed of as risk waste immediately after use. | ||
Where off-site catering is used, a suitable area for receiving should be provided. Space will be required for sorting meals for distribution and collecting and storing dirty dishes, washing dirty dishes, if necessary, and disposing of left-over food and disposable containers and utensils. The size of the areas required for this will depend on the number of meals delivered. | Where off-site catering is used, a suitable area for receiving should be provided. Space will be required for sorting meals for distribution and collecting and storing dirty dishes, washing dirty dishes, if necessary, and disposing of left-over food and disposable containers and utensils. The size of the areas required for this will depend on the number of meals delivered. | ||
| + | |||
=== CSSD === | === CSSD === | ||
The primary function of a Central Sterile Supply Department (CSSD) is to provide an efficient, economic, continuous and quality supply of disinfected and sterilised items, when needed, to all patient-care service points in the ACS, and to receive returned contaminated items for cleaning. | The primary function of a Central Sterile Supply Department (CSSD) is to provide an efficient, economic, continuous and quality supply of disinfected and sterilised items, when needed, to all patient-care service points in the ACS, and to receive returned contaminated items for cleaning. | ||
Revision as of 15:07, 5 May 2020
Return to Infrastructure Guidance for COVID-19
Contents
- 1 Infrastructure Minimum Guidelines for Alternate Care Sites for COVID-19
- 2 Purpose and Approach
- 3 Scope and Assumptions
- 4 Status Quo
- 5 Strategic Approach
- 6 Typology Dictates (Case Studies)
- 7 ACS Infrastructure Planning
- 8 Site Selection
- 9 Infection Prevention and Control
- 9.1 Transmission-based precautions
- 9.2 Additional precautions
- 9.3 Spatial strategies for infection prevention and control
- 9.4 Restricted access and zone control
- 9.5 Site layout and master-planning
- 9.6 Operational Strategies
- 9.7 Personal protection
- 9.8 General transmission mitigation
- 9.9 Notes and References:
- 10 Health, Safety and Well-being
- 11 Schedule of Accommodation
- 12 Clinical Services
- 13 Logistical Services
- 14 Support Services
- 15 Environmental Controls
- 16 General Indoor Environment Conditions
- 17 Engineering Services
- 18 Additional Resources
- 19 References
Infrastructure Minimum Guidelines for Alternate Care Sites for COVID-19
This guidance work was initiated under the project titled:
Reducing Nosocomial and Community-Acquired Tuberculosis by Strengthening the Capacity of the South African Department of Health to Improve Implementation of Infection Control and Waste Management at All Levels of the Health System Under the President's Emergency Plan for AIDS Relief (PEPFAR)
Purpose and Approach
The global pandemic of COVID-19 caused by the coronavirus, SARS-CoV-2 is likely to result in a surge in need for medical care for Severe Acute Respiratory Syndrome (SARS) in South Africa. Considering the course of the pandemic in other countries, it is anticipated that South African hospitals will not have sufficient capacity to cope with the surge of persons requiring medical attention and that surge capacity via alternate care sites (ACS) will need to be established.
Surge capacity, contemplated here is not the frequent emergency department overcrowding experienced by healthcare facilities (e.g. Friday/Saturday night emergencies) or local casualty emergency that might overcrowd nearby facilities and have little to no impact on the overall healthcare delivery system. It is when a catastrophic event occurs and the affected population seek medical care from existing local healthcare facilities, causing healthcare infrastructure to become exhausted due to excess in demand. During a healthcare surge, the standard of care will shift from focusing on patient-based outcomes to population-based outcomes, and providers should anticipate “a shift to providing care and allocating scarce equipment, supplies and personnel in a way that saves the largest number of lives in contrast to the traditional focus on saving individuals.”[1]
Surge capacity can be temporarily established in non-traditional environments, such as hotels, exhibition halls, community halls, and as field hospitals, on open spaces.
In the context of this document, a quarantine site is a facility for patients who do not require continuous professional medical care, while an ACS is defined as a temporary facility that can provide continuous medical care for SARS. This document provides principles and considerations, high-level guidance for minimum requirements and examples for ACS.
While an extensive set of health facility guidelines does exist[2], these are applicable for conventional facilities and thus include services and guidelines that are not necessarily relevant to the treatment of a novel, highly infectious pathogen, with pandemic effects. Moreover, these do not provide well for the rapid and temporary establishment of facilities.
In order to formulate high-level guidance, as invited by Business for South Africa, the team reached out to professional industry bodies for inputs, in particular the South African Institute for Architects (SAIA), The Gauteng Institute for Architects (GiFA) Gauteng Institute for Architecture and the South African Federation of Hospital Engineering (SAFHE), by inviting input via a 36-hour research charrette. Relevant historical and contemporary literature was consulted, precedents identified and critically reviewed. An interaction with the team at Wuhan responsible for makeshift hospitals and emergency infectious diseases hospitals, Central-South Architectural Design Institute, was arranged with assistance of the Chinese Embassy. Material from the Infrastructure Unit System Support (IUSS), international literature and guidance and input gathered from the broader architectural, engineering and healthcare professional communities was synthesised and moderated by the CSIR team. The draft was reviewed by an expert review panel.
Acknowledgement of contribution
The contributions to the initial version of this were gratefully received. A list of these contributors can be viewed here..
New contributions are eagerly encouraged along with debate and discussion on the discussion forum. tab above.
Notes and References:
Scope and Assumptions
ACSs as discussed in this document are dedicated, temporary facilities for triage, testing, diagnosis, further referral and treatment of persons:
- suspected of having contracted SARS-CoV-2, (persons under investigation (PUIs)), who are symptomatic and/or are awaiting results,
- or are confirmed to be infected.
ACS will accommodate a variety of clinical, logistical, support and auxiliary services associated with the render of care. ACS will currently not be licensed to provide healthcare services. Since the ACS will operate in a non-healthcare facility, it cannot fully replace a hospital setting and its prime objective is to manage the patient load until the local healthcare system can meet demands.
Exclusions
Quarantine facilities are accommodation facilities where a member of the community can remain for the duration of their isolation period. This is typically temporary housing for a cohort of people who do not need intensive medical attention but who cannot stay at home. Patients can take care of themselves and need limited monitoring by medical staff. Quarantine: Containing presumptive-case patients from each other and the general population. Quarantine facilities – that is for asymptomatic persons who are in the community in self- or imposed isolation, but not displaying symptoms, or who are symptomatic, but are able to safely recover without clinical intervention and do not need continuous medical observation are not considered in this document.
Service regime
The following assumptions are made with respect to services under consideration.
- Temporary - limited to the part of the pandemic when the “conventional” hospital platform cannot meet demand. To be dismantled, thereafter.
- Uncomplicated, dedicated COVID-19 care is to be prioritised for ACS.
- Patients with comorbidities, paediatrics will be prioritised for conventional facilities, and only accommodated in ACS as a matter of last resort.
- 24 hour, 7 days a week operations.
Assumed mechanism of transmission
Transmission of SARS-CoV-2 is understood to be from person to person firstly by droplet transmission, then by the contact route and finally via airborne transmission during or following mechanical aerosolisation. Water transmission risks are minor, occurring in special Fecal-oral circumstances. Reclassification of transmission mechanisms may nullify some of the approaches presented in this guidance.
A call for strategic coordination
This document focusses on infrastructure requirements. These provisions are meaningless without staffing, equipping and resourcing. Whilst staffing, equipping and resourcing are not the focus of this document, these are likely to emerge as key constraining features. Resource constraints are likely to become acute during this pandemic. Doctors and nurses are already in critical short supply in South Africa and internationally, and are themselves susceptible to COVID-19 infection. Equipment and consumables are in short supply with heightened global demand, reduced manufacturing capacity and limits in trade flows. This necessitates strategic coordination, proactive planning, options appraisal and prioritisation.
Notes and References:
Status Quo
Rationale and transmission status
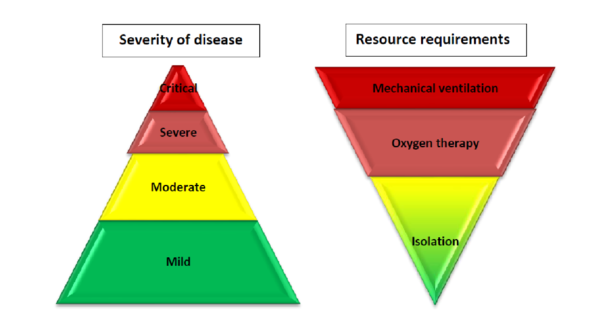
According to the World Health Organisation (WHO), based on the largest cohort of COVID-19 patients, about 40% of patients with COVID-19 may have mild disease, where treatment is mostly symptomatic and does not require inpatient care. About 40% of patients have moderate disease that may require inpatient care; 15% of patients will have severe disease that requires oxygen therapy or other inpatient interventions, and about 5% have critical disease that requires the patient to receive mechanical ventilation. However, the evolution of the outbreak in some countries has shown a higher proportion of severe and critical cases and the need to rapidly increase surge capacity to prevent rapid exhaustion of biomedical supplies and staff. In some countries, doubling rates of cases every three days has been observed[1]
South Africa has a high burden of disease, with a high prevalence of HIV and TB. Although evidence is yet to emerge of the effect of SARS-CoV-2 on a population with these pre-existing conditions, there is reason to proceed with caution[2]. There is a potential direct and indirect benefit of ACS to people living with HIV and TB, as well as to general public health and the health system preservation.
With the travel lockdown in place, and continued transmission, it appears that South Africa is on the cusp between cluster transmission and community transmission according to WHO’s classification, shown in the table below, indicating that preparation should include temporary hospital facilities and mass critical care.
Key clinical and infection control activities for different transmission scenarios [3]
| No Case | Sporadic Case | Clusters of Cases | Community Transmission | |
|---|---|---|---|---|
| Faculty Space, Including for Transmission | Usual Space. Enhanced Screening and triage at all points of first access to the health system | Dedicated COVID-19 patient care areas within health facility (e.g. infectious disease ward, isolation rooms in emergency or ICU wards). | More patient care areas re-purposed for COVID-19 within the health system, especially for severe cases | Expanded care for severe cases in new hospitals or temporary hospital facilities |
| Staff | Usual space. Enhanced screening and triage at all points of first access to the health system | Dedicated COVID-19 patient care areas within health facility (e.g. infectious disease ward, isolation rooms in emergency or ICU wards) | More patient care areas repurposed for COVID-19 within the health system, especially for severe cases | Expanded care for severe cases in new hospitals or temporary hospital facilities |
| Supplies |
|
|
|
|
| Standard of Care | Usual care with enhanced awareness and recognition of immediate needs for first COVID-19 patients | Usual care and treatment for all patients, including those with COVID-19 | Identify context-relevant core services. Shift service delivery platforms. Consider reduction in elective patient encounters, including elective surgical procedures. | Mass critical care (e.g. open ICU for cohorted patients). |
| Care areas expansion | No requirements for expansion | Designate 10 beds per suspected COVID-19 case | Expand COVID-19 patient care areas by a factor of 35 | Expand COVID-19 patient care areas by a factor of 58 |
Quantification of need
At this time there are various parallel initiatives aimed at forecasting the South African epidemic, quantifying the projected need for facilities, and a shortfall in existing capacity. At this time, there is no consensus on this. This section will be updated as further data becomes available. ACS will prioritise mild to moderately affected COVID-19 patients where basic, targeted medical care will be provided. Should patients’ needs evolve, requiring escalation of care, then the transfer of patients from ACS sites to conventional sites of care will be needed as a matter of course, bringing with it logistical challenges and risks. In the event that the conventional hospital platform is unable to cope, ACS will have pressure to provide care for severe and critical patients, and finally for patients with comorbidity and special requirements, such as paediatrics, persons living with HIV (PLHIV), TB patients and pregnant women. The following pragmatic approach, aligned with the WHO recommended strategic approach, is suggested.
- ACS should preferably be identified with space for expansion.
- The set-up should be done so that levels of care can be upgraded to higher levels of uncomplicated care.
- A secondary upgrade for more diverse package of care may become necessary.
Notes and References:
- ↑ WHO-2019-nCoV-HCF_operations-2020, https://apps.who.int/iris/bitstream/handle/10665/331492/WHO-2019-nCoV-HCF_operations-2020.1-eng.pdf
- ↑ The Conversation 2020, https://theconversation.com/tb-hiv-and-COVID-19-urgent-questions-as-three-epidemics-collide-134554
- ↑ WHO 2020, https://apps.who.int/iris/handle/10665/331492
Strategic Approach
According to WHO, clinical interventions must be put into place immediately and then scaled up according to the epidemiologic profile.
Under this declared state of disaster, the clinical care strategy which cannot be accommodated within existing facilities, can, on a temporary basis be hosted in ACS:
- Within and around existing healthcare facilities, via reconfiguration and/or augmentation.
- In existing non-healthcare buildings suitable for repurposing, such as universities, hotels and conference centres, warehouses, gyms, hostels etc.
- On open fields, including paved parking areas with rapidly constructed, dismountable structures, such as modular tented structures or using rapid modular construction techniques.
ACS will provide isolation, general (non-acute) care for patients with mild to moderate symptoms and as required, acute care for patients with severe symptoms. Containing confirmed-case patients from general population. Confirmed-case patients can be housed together en masse, while presumptive-case patients must be individually quarantined.
As shown in the WHO Strategic approach to clinical care, the WHO recommends a range of services to meet patient need (Citation needed). General (non-acute) care ACS model is designed for minimal acuity patients requiring minimal activities of daily living support (e.g. COVID-positive with minimal symptoms or require <2L of oxygen). Acute care ACS model is designed for higher acuity patients requiring closer monitoring or respiratory support (e.g. COVID-positive with pneumonia or respiratory distress requiring ventilator support). Paediatric patients are to be accommodated in separate wards, where strictly controlled visitation may be allowed.
As a preliminary estimate, the following ratios of service is proposed:
| Case severity, risk factors[Notes 1][Note 1] | Recommendations |
|---|---|
| Mild | Patient should be instructed to self-isolate and contact COVID-19 information line for advice on testing
and referral. |
| Moderate, with no risk factors | Test suspected COVID-19 cases according to diagnostic strategy. Isolation/ cohorting in:
(i.e. adjacent COVID-19 designated health post/EMT-type 1, telemedicine)
|
| Moderate, with risk factors | Patient should be instructed to self-isolate and call COVID-19 hotline for emergency referral as soon as possible |
| Severe | Hospitalization for isolation (or cohorting) and inpatient treatment. |
| Critical | Hospitalization for isolation (or cohorting) and inpatient treatment. |
Notes and References:
- ↑ Known risk factors for severe COVID-19: age over 60 years, hypertension, diabetes, cardiovascular disease, chronic respiratory disease, immunocompromising conditions.
Typology Dictates (Case Studies)

To meet the requirements set out in this guidance, prospective “host” sites should be carefully evaluated. The type of “host” site selected will strongly influence or dictate the choice of ACS service model.
No site is likely to meet all requirements and recommendations set out in this document. Adaptations and compromises will be necessary. Services should be provided on site where it is pragmatic to do so, for example where similar services are provided. Outsourcing can also be practical/feasible for some services, such as laboratory services, catering and laundry, provided suitable logistical arrangements can be made.
Some typological responses and service models are set out in precedent examples, shown below. The examples demonstrate that a variety of host settings are workable, provided that the appropriate utility can be contrived. Other than in metroplitan areas, co-location of ACS on the premises of, or adjacent to existing halthcare facilities will often be preferable because this is where intensivists and specialist clinical staffing will be available, and support services may be well established. Nevertheless, augmenting capacity at existing facilities should take into account current workloads and capacity to ensure that the COVID-19 surge disrupts normal service provision as little as possible, including continuity of care for patients with chronic conditions and TB and HIV patients.
Notes and References
- ↑ Coronavirus: Building NHS Nightingale Hospital London, 2020 https://www.bbc.com/news
ACS Infrastructure Planning
Establishing a team
A planning team should be formalised to establish the minimum planning and operational requirements for the ACS and to liaise with the local community. The team should include individuals with expertise in the following areas (ideally with knowledge of healthcare delivery under emergency conditions):
- Disaster response / emergency management coordination,
- Clinical care and staffing,
- Infection Prevention and Control practitioners must be involved in all stages of planning, commissioning, in-use, and decommissioning of the facility
- Facility set-up, operations and management,
- Security,
- Transport (patient, staff),
- Engineering and project management,
- Procurement and coordination of supplies, equipment and pharmaceuticals, and
- Community liaison to ensure that concerns of the adjacent population on understood an addressed.
It is important to ensure compliance with health, safety and building regulations, by ensuring the involvement of relevant local authorities. Stakeholder engagement should be formally documented. Concerns and grievances should be systematically addressed.
Structural integrity and operational responsibility
Structural modifications: ACSs are for temporary use and any modifications necessary for the establishment of the clinical and associated support services should be undertaken with minimum invasiveness to the structure so that restoration to the original function is considered.
Competent person: All structure, water, electricity, fire, gas and infection prevention and control installations, whether temporary or permanent must be designed and installed by competent persons. Any modification to any existing structure must be undertaken with prior knowledge and express approval of a duly appointed competent person (such as a registered professional engineer or architect) who is to take responsibility to ensure structural integrity. Competent persons should be explicitly appraised of the nature of services to be rendered, have access to multi-disciplinary specialist support as required and have professional indemnity insurance covering the scope of work. Competent persons shall ensure that all temporary structures are adequately specified and fastened, and safe for use for the purpose they are installed.
Asset responsibility: Unless otherwise agreed, equipment provided to the ACS, will be presumed to be the property and responsibility of the supplier, (including consumables and maintenance) until duly authorised evidence of asset transfer is documented.
Integrity and responsibility
Structural modifications: ACSs are for temporary use and any modifications necessary for the establishment of the clinical and associated support services should be undertaken with minimum invasiveness to the structure so that restoration to the original function is considered.
Competent person: All structure, water, electricity, fire, gas and infection prevention and control installations, whether temporary or permanent must be designed and installed by competent persons. Any modification to any existing structure must be undertaken with prior knowledge and express approval of a duly appointed competent person (such as a registered professional engineer or architect) who is to take responsibility to ensure structural integrity. Competent persons should be explicitly appraised of the nature of services to be rendered, have access to multi-disciplinary specialist support as required and have professional indemnity insurance covering the scope of work. Competent persons shall ensure that all temporary structures are adequately specified and fastened, and safe for use for the purpose they are installed.
Asset responsibility: Unless otherwise agreed, equipment provided to the ACS, will be presumed to be the property and responsibility of the supplier, (including consumables and maintenance) until duly authorised evidence of asset transfer is documented.
Decommissioning: Decommissioning of the facility shall be assigned to the competent person discussed above. All residual structures upon decommissioning shall comply with the National Building Regulations. Upon decommissioning, removal of equipment shall be the responsibility of the owner. An infection prevention and control practitioner should be engaged in the decommissioning phase to oversee terminal cleaning and disinfection of equipment and premises.
Closure: Once all patients can be safely discharged or transported back to existing facilities for continued care and there are no ongoing healthcare surge capacity needs, the ACS can be closed. Shut down of an ACS will require decommissioning, identification of new homes or storage for equipment, and termination of ongoing contracts or arrangements. Shut down should be expedited so that the facility can quickly be returned to the control of the existing owners and returned to its usual function.
Action checklist items for ACS closure should include, but not be limited to, the following:
- conduct a site walk-through with the facility owner when shutdown activities are completed to ensure that terminal cleaning and disinfection of supplies and premises, removal of equipment and supplies, and other surge closure activities have been completed to the owner’s satisfaction.
- perform medical records storage procedures.
Site Selection
When selecting a site, the National Department of Health COVID-19 - Guideline Room List for Planning a Temporary Hospital can be utilised to determine whether the site is suitable for a 100, 1000 or 2000 bed facility, as required. The following indicative minimum site sizes are needed:
- 100 Bed ACS/ hospital conversion, requires ± 4 300 m2
- 1000 Bed ACS/ hospital conversion, requires ± 17 600 m2
Evaluation should be done by examining plans (if available), satellite images, drone images, scans and by physical inspection (walkabout). A comprehensive photographic survey should be undertaken and retained for record purposes on the site inspection. This will serve as an audit record and may assist in returning the site to its original function on ACS decommissioning and closure. When scrutinising documents and conducting site inspections to confirm the suitability of a site to host an ACS, the following criteria should be taken into account.
Criteria
- Affordability (costs, including operational costs known and budget identified),
- Sufficient physical space and capacity to house the immediate need, with the potential to accommodate physical space requirements. For example, open site should not be sloping,
- Legal rights and encumbrances, including renewal opportunity,
- Good access to highway and main roads,
- Well secured perimeter and limited controlled access points,
- Proximity to other hospitals and care sites,
- Accessibility for key staff and public transport,
- Good vehicular access around the site to set up temporary equipment such as back-up generators,
- Free from clear and present danger,
- Outside attenuation zones, floodplains,
- Outside high wind zones,
- Structure in good repair,
- Effective onsite facilities management team who understand how systems work,
- Potential for expansion, if required,
- Access to sufficient capacity for
- potable water,
- adequate sewage,
- telephone,
- internet access with sufficient bandwidth,
- electricity,
- A zone for cleaning, disinfection, and decontamination of equipment at least 15 metres away from occupied areas with access to water, a hard impervious surface and drying areas in the sun, with runoff discharge into the sewer and not into marine ecosystems or the environment. Include area for cleaning and storage of cleaning equipment.
- Likelihood of acceptance of hosting an ACS by the adjacent and local community
Desirable
- Durable, cleanable surfaces,
- Large open spaces that can be converted to accommodate temporary structures,
- Good ventilation,
- On-site kitchen and laundry,
- Housekeeping staff (chemical and equipment storage, lockers, rest facility, administration office),
- Space conducive for staff respite area and locker rooms,
- Amenities with universal access,
- Fire protection safety and equipment,
- Elevator access for patients if the building has more than one floor,
- Capacity for expansion, and
- Accessible to at least two roadways to provide continued access in the event that one roadway becomes blocked on inaccessible.
Infection Prevention and Control
General guidance for COVID-19 Infection Prevention and Control can be accessed here
Infection prevention and control in the context of COVID-19 should respond to transmission routes of primary concern for the pathogen of interest (droplet and contact transmission, and management of risk waste) as well as infection risk of a general nature (water and sewerage, airborne transmission – under high TB/HIV burden, and general waste). In addition to satisfying standard precautions for all patient care, transmission-based precautions should focus on three pillars: exposure reduction by spatial configuration, operational strategies, and personal protection.
Transmission-based precautions
Droplet and contact spread: Transmission of SARS-CoV-2 virus occurs via droplet and contact spread. The virus has been shown to persist on surfaces for extended periods of time and is known to be efficient at infecting people.
Medical waste and linen: As SARS-CoV-02 is carried in body fluids and faecal matter, disposal of contaminated items (tissues) and cleaning regimes (spaces, garments, linen) should be accommodated carefully in the workflow design and infrastructure provision. A site-specific waste management plan should be formulated in accordance with a site-specific waste management plan with reference to SANS 10248.
Airborne transmission: Under exceptional circumstances, the risk of airborne transmission arises for SARS-CoV-2, as detailed below.
| Airborne Transmission Risk Factors |
|---|
|
As SARS-CoV-2 is not considered airborne, respiratory protection against airborne transmission is not considered necessary, except where aerosolisation of particles may be a risk. According to the CDC
According to doctors in the field also when performing
|
South Africa has a high prevalence of TB and HIV, and therefore, although the risk of COVID-19 transmission via the airborne route is not paramount, there is a high likelihood that undiagnosed TB infectious patients may present at the ACS for treatment. TB triage may be challenging in the ACS as there are symptoms in common (coughing) with COVID-19. This country-specific risk is taken into account in this guidance
Additional precautions
Water and sewerage contamination: The International Water Association concluded that water and sewerage contamination is not considered to be a key risk factor for COVID-19. The panel expressed concern for “how waste and specifically wastewater (medical) would be handled by places (e.g., hostels, hotels) that are used to serve as interim COVID-19 quarantine or testing facilities or accommodation ([ACS]. These are places other than hospitals that are used in the interim for such purposes and do not usually handle wastewater from medical settings. Such facilities should be monitored carefully.”
Spatial strategies for infection prevention and control
Restricted access and zone control
The site will be arranged to establish clear zoning, with a clear restricted zone protocol and access protection. Zones should be deemed to be "contaminated" or "uncontaminated" with clear transition areas between them.
- Contaminated zones
- (also known as "dirty areas") are areas occupied by COVID-19 infected persons, PUIs, equipment, materials and supplies which have come into contact with such persons without yet undergoing a decontamination procedure. These areas will include patient ward areas and ablutions, patient admissions (including ambulance drop-off) and the associated clinical areas. Staff rest and dining facilities should be outside the contaminated zone. Limited stock for immediate use should be kept in the contaminated zone. Layout designs should consolidate contaminated zones as far as reasonable, and avoid uncontaminated zones as islands in contaminated zones.
- Uncontaminated zones
- (also known as "clean areas") are areas not generally occupied by PUIs or confirmed COVID-19 infected persons. Equipment, materials and supplies in these areas have not yet come into contact with contaminated zones or have undergone a decontamination procedure. These will include clinical management planning rooms, stock rooms, bulk stores, pharmacy, laboratory areas, kitchen and laundry.
- Transition zones
- (also known as intermediate zone) are the spaces through which transfer of people and goods from uncontaminated to decontaminated zones, and vice versa, occur. Materials from the contaminated zone should be decontaminated or contained in the transition zone. The transfer of goods and persons should be highly ritualised and, as far as possible, traffic across transition zones should be minimised. Transition areas should be strategically located to serve this function. Separation of in-going and out-going transfer of goods and persons is preferable. Transition areas include ambulance, trolley decontamination, CSSD, laundry and waste bagging areas, patient locker area and staff change areas with spaces for donning and doffing of PPE.
Site layout and master-planning
Spatial configuration and layout can ensure unnecessary cross-over of function is avoided. This entails the systematic separation of functions and the managed transition between activities to facilitate consistency of care, an orderly, efficient work environment, less waste and reduced risk for improved outcomes. To achieve this, functional relationships should first be considered at the site level before being considered at the building level.
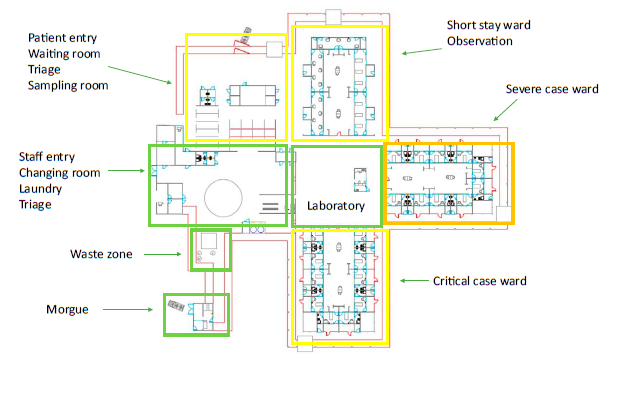
Layout for a SARS facility, clustering functions with minimised cross-over [1]
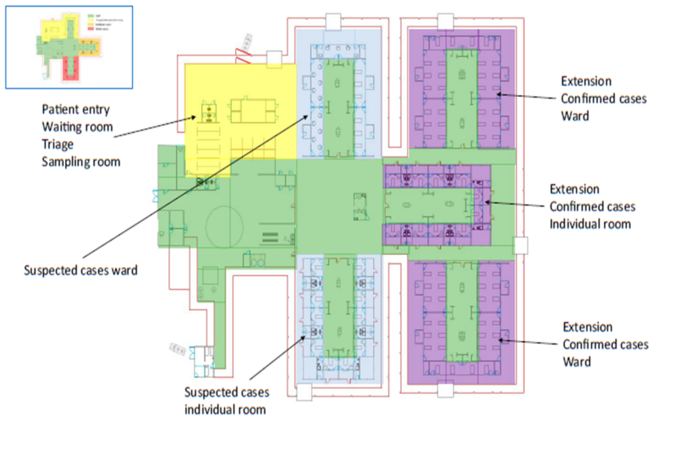
Layout for a Patient cohorting strategy [2]
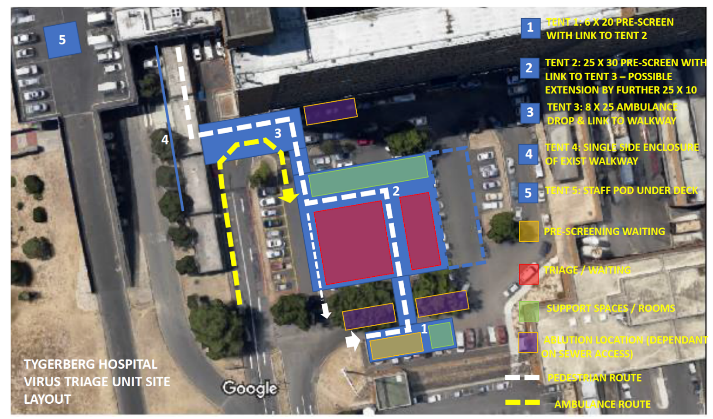
Tygerberg Hospital virus triage unit site layout [3]
The WHO's Clustering Layout [1] and Tygerberg Hospital virus triage unit[3] show worked examples of building and site layouts, which are configured with these principles, respectively. Cohorting is defined as clustering patients with similar or compatible clinical needs together for risk reduction, acuity, efficiency and quality management, as illustrated in WHO's Cohorting Layout [2]
Workflow
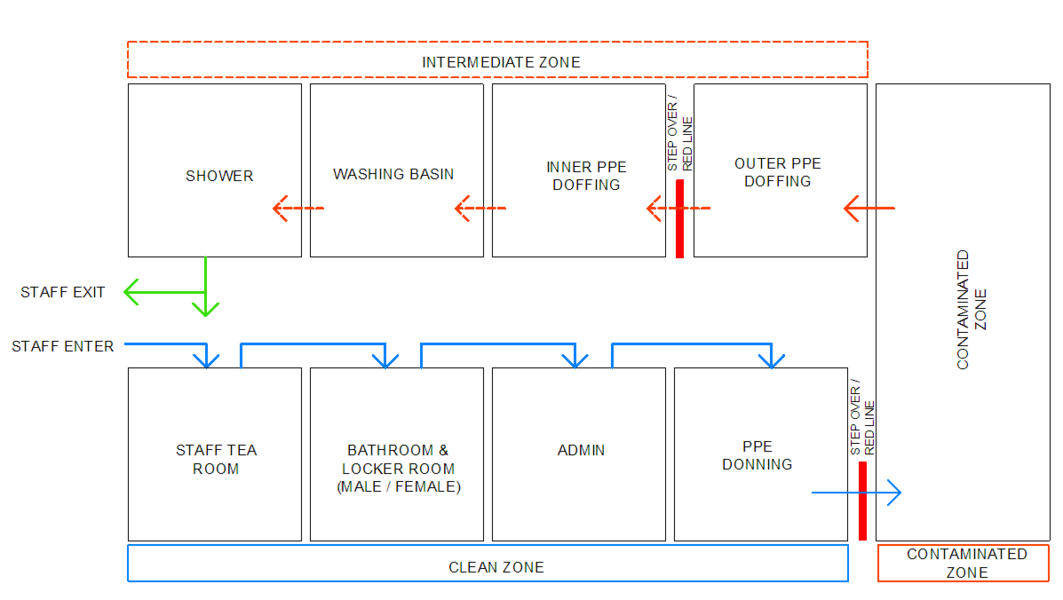
Within individual functional zones, the workflow activities can be arranged to proceed from clean procedures to contaminated procedures. In the example below, the staff arrival, PPE donning, doffing and patient flows are worked to have controlled interaction and minimised cross-over
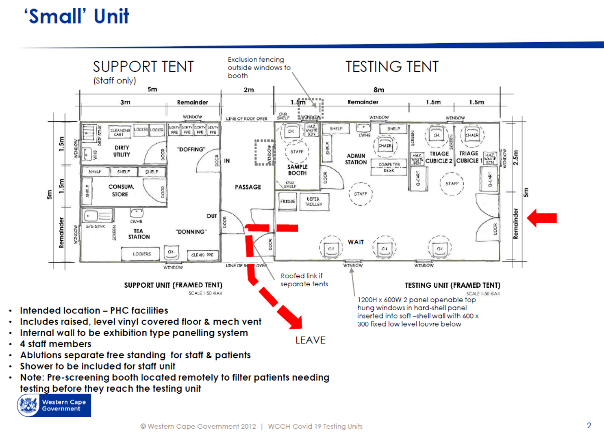
As far as possible, a single direction flow of clean to dirty is recommended for all processes: support services, supply and waste. The Small ACS unit workflow diagram[4] illustrates the recommended separation of access and exit, separate waiting seats, for persons who may be COVID-19 infected. Separate spaces are provided for donning and doffing PPE. Staff change areas are provided.
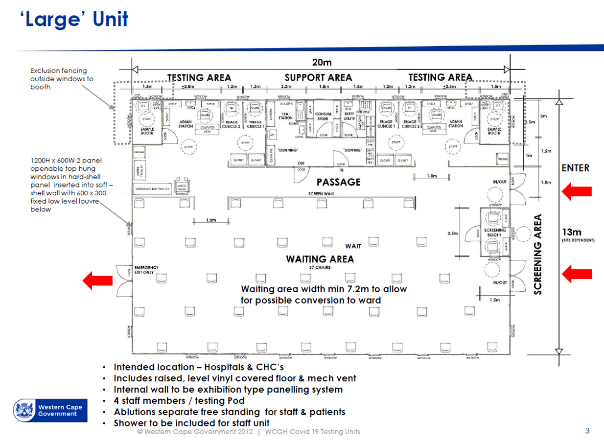
In the Large ACS unit workflow diagram [4], there is a clear separation between staff areas and patient areas. Waiting seats are set far apart to reduce transmission risk. Staff change rooms are provided near the point of entry to the facility for staff to change from street clothes into medical work clothes. To prevent work clothes worn inside the facility from contaminating street clothes, these are kept in separate lockers. A step-over barrier from dirty to clean sides of the change room is helpful to enforce a mind-set of avoiding cross-contamination. Bins for contaminated garments are to be provided in change rooms. Shower facilities are to be provided for staff.
Operational Strategies
Cleaning, disinfection and decontamination
Surface and substrate specification, and detailing of all areas should, as far as possible, allow for frequent:
- Cleaning with detergent and water.
- Disinfection with 75% alcohol solution (metal surfaces).
- Sodium hypochlorite (1,000 ppm)/ Household bleach.
- Disinfectants listed on the EPA List N[5] (for non-critical environmental cleaning).
- High-intensity ultraviolet surface disinfection (UV-C).
- Decontamination and sterilisation of clinical equipment.
Goods and waste management
Remove any unnecessary furniture, equipment and paraphernalia from all patient care and clinical areas. Provide a clear, secure space for waste management. Any potentially infectious waste materials generated at the ACS should be considered and treated as medical waste (health care risk waste). The applicable legislation is:
- The National and Provincial Health Care Risk Waste Management Regulations.
- National Department of Health COVID-19 Environmental Health Guidelines.[6]
Waste disposal bins should be positioned near the exit inside each patient rooms or wards to make it easy for staff to discard PPE after removal, prior to exiting the room, or before providing care for another patient in the same room.
Materials and finishes
Floor materials must be:
- Level,
- Free of dust and oil,
- Impervious and smooth,
- Slip-resistant in wet areas (e.g. patient ablutions).
Smooth, cement screed floors are acceptable. Where hosting facilities have carpeted areas, a risk assessment of factors such as durability, hygiene and decontamination needs to be conducted. In cases where the acceptance of carpeted flooring is contradicted (but other factors make it a compelling option), temporary floor finishes or covering can be investigated.
Personal protection
Hand sanitation
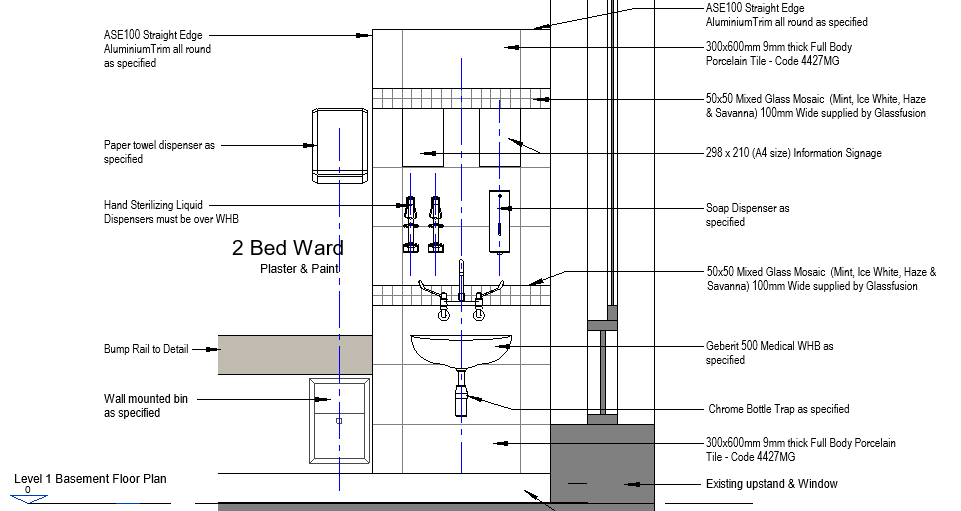
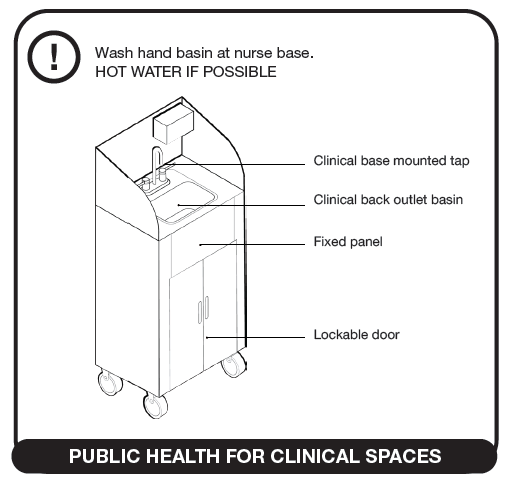
Where wash-hand basins are not provided, clinical wash-hand basins should be installed, at the minimum rate of provision of one wash-hand basin per 5 beds. Clinical wash-hand basins (see figure below) have a variety of features not present in standard wash-hand basins, which are preferable for infection prevention and control. Where standard wash-hand basins are provided, an upgrade is not necessary. In all cases, there should be no surfaces and no clutter, equipment or supplies in the vicinity of wash-hand basins, including surgical gloves.
Where wash-hand basins are not available, portable units can be used, as shown above[8]. Mounted brackets for hand sanitisers are to be provided for every two beds, preferably mounted near the foot rather than the head of the bed and at all common touch points such as entry points at ablution facilities, linen room, sluice, storerooms, medicine rooms/cupboards, near refrigerators, telephones, light switches, at entry/exit doors, etc.
Personal protective equipment
Donning and doffing points for personal protective equipment, and convenient, safe disposal of consumables to be placed at critical key points when entering patient areas.
General transmission mitigation
Water and sanitation
To comply with National Building Regulations; Hazardous Biological Agents Regulations and National Department of Health COVID-19 Environmental Health Guidelines[6].
Droplet aerosolisation
When designating areas for procedures during which aerosolization and airborne transmission risk is high, the building ventilation must be carefully considered to take into account downstream risks. In particular, consider to where potentially contaminated air, arising from aerosol-generating procedures, is exhausted. In general, air exhausted directly to the outside is diluted and considered safe, unless there are openings to occupied spaces near the exhaust air outlet.
In the event that potentially occupied spaces will receive partially diluted or undiluted contaminated air, or where this is indeterminate, the aerosolising activity should be designated to an alternate area. In the event that an alternative is not available, some treatment regime (air filtration or air disinfection) is necessary.
In most naturally ventilated settings, the airflow direction between zones may fluctuate according to the wind direction. Such high-risk spaces should not be adjacent to spaces with high susceptibility rates, such as PUI areas and uncontaminated areas. Contaminated areas should not be directly adjacent to clean areas unless mechanically ventilated.
Guidance on COVID-19 building ventilation design is provided here.
Notes and References:
- ↑ 1.0 1.1 WHO, 2020 Severe Acute Respiratory Infections Treatment Centre
- ↑ 2.0 2.1 WHO, 2020 Severe Acute Respiratory Infections Treatment Centre
- ↑ 3.0 3.1 Western Cape Provincial Government, 2020 a
- ↑ 4.0 4.1 4.2 4.3 Western Cape Provincial Government, 2020 b
- ↑ The United States Environmental Protection Agency, List N: Disinfectants for Use Against SARS-CoV-2 (Last Visited 2020)
- ↑ 6.0 6.1 National Department of Health COVID-19 Environmental Health Guidelines [1]
- ↑ de Jager 2020
- ↑ 8.0 8.1 BDP 2020, NHS nightingale instruction manual, [http://www.bdp.com/globalassets/projects/nhs-nightingale-hospital/nhs-nightingale-instruction-manual.pdf http://www.bdp.com/globalassets/projects/nhs-nightingale-hospital/nhs-nightingale-instruction-manual.pdf
Health, Safety and Well-being
In addition to the infection prevention and control measures discussed above, the following should be provided for health, safety and well-being.
General provisions
- Minimised and controlled entry and exit points, with suitable control.
- Clearly identified accessible and marked routes for patients, staff, goods and waste.
- Clear designation of restricted zones.
Site level provisions
- Safe staff parking and arrival of staff via planned and public transport.
- Clearly demarcated parking for people with disabilities.
- Arrival and departure point for patients via public transport, passenger vehicles, and emergency service.
- Limited safe patient parking.
- Supply of goods and removal of waste.
Within and between buildings
- Entrances with a clear opening width (CoW) of at least 900mm.
- Routes with a minimum width of 2 000mm free of hazards, for example, rubbish bins.
- All clinical, patient and support areas to be accessible by trolley.
Ramps should be of stable construction, capable of sustaining a mass of 300kg. They should incorporate side lips and the surface should be slip-resistant. Gradients should be as gentle as the circumstances allow. (Recommended maximum 1:20).
Small changes in floor levels are not desirable, but where these exist are to be clearly marked with reflective paint/ tape, and lit at night
Elevators between different floors, where patients need access (The recommended minimum lift size for patient trolley/stretcher movement is 1 400mm × 2 400mm, however, this may not be possible).
Pathways to be lit at night, where used at night.
Staircases must be well-lit at night with non-slip surfaces and secure balustrades.
Doors, Double doors and automated or push-operated doors to all clinical areas are to be preferred, where these are newly installed or able to be retrofitted. Door closers are to be disabled, where not necessary to reduce touch surfaces. Hand sanitisers to be provided at where high touch common surfaces occur (e.g. wall-mounted at doorways).
Signage
The appropriate level of information to facilitate legibility, orientation and wayfinding. Minimum standards, signage to be:
- Clearly visible, simple font, font size, contrasting colours, placed in the field of vision
- Washable
- Comprehensive safety signage - fire signage (exits, equipment etc.)
- Restricted areas clearly marked
- Identification signage - each patient space to be allocated a unique number and a whiteboard or perspex sheet for writing the patient’s name
Signs should be posted immediately outside of patient rooms indicating appropriate IPC precautions and required personal protective equipment (PPE). Signage of a temporary nature can be provided on laminated white A4 sheets attached eye-level. The text should be black sans-serif (for instance Arial) text at least 40point size and centrally positioned on the sheet. Detailed guidance on signage is provided in IUSS Inclusive environments.
Safety and security
Upon identification of the ACS host site, a team should be convened to conduct a multidisciplinary safety and security analysis. These critical team members need to form the working committee responsible for undertaking the detailed assessment of the existing facility’s security. They should analyse data about the security system’s condition and review existing security concerns or issues that are reasonably likely to become concerns in the near future. The figure below represents a five-zone approach to security, which is a recommended, systematic approach to security.
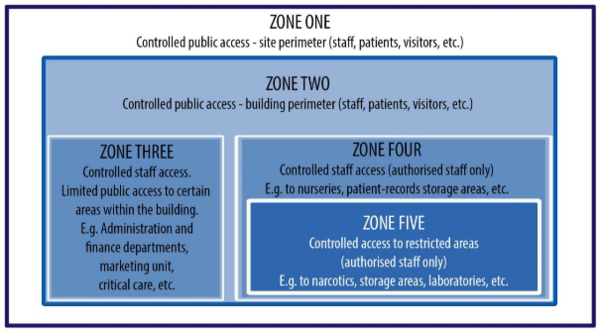
The security strategy should take into account that whilst clinical services and some logistical and support services will be required 24 hours a day, seven days per week, some support services, logistics services and auxiliary services may only be operational for the minimum periods required to meet demand. These functional elements should be capable of being secured, for example over weekends and at night, as the case may be. Detailed guidance is available in IUSS Hospital design principles - Security [1]
Comfort and dignity
Supplemental heating: Patient health and comfort are dependent on, amongst others, maintaining body temperature. The ACS structural technology must be selected to achieve the general indoor environment conditions discussed in a subsequent section for all clinical and occupied areas. As we are moving into South African winter supplemental heating may be required, especially in the evenings, in order to avoid hypothermia. Use of fans, bar, radiator or gas heaters should be prohibited. Unless clinical areas can be maintained above 18 degrees centigrade, patients should each have an infrared heater available, in addition to blankets. Personal/ donated blankets can be considered if they are laundered first and could be destroyed upon discharge. Mobile screens should be available to provide privacy where necessary (e.g. during consultations or procedures).
Some solutions which address patient privacy and dignity are depicted above.
Notes
Schedule of Accommodation
Based on clinical needs of the ACS, a schedule of accommodation can be crafted capturing the clinical, clinical, logistical, support and auxiliary services associated with the render of care. When deriving a schedule of accommodation, the National Department of Health COVID-19 - Guideline Room List for Planning a Temporary Hospital tool can be used. Functions to be accommodated are:
Clinical services: Triage, rapid assessment of persons entering the facility, to expeditiously identify and render the appropriate service. Admissions and registration. Inpatient accommodation is to be organised according to cohorting principles, discussed above. Testing and diagnostics, including laboratories and x-ray. Safe storage and dispensing of drugs to patients.Offices for clinical administration and clinical planning meeting rooms in the clean zone are needed.
Logistical services: Logistical services will entail management of flows of people, goods, services and information to and from the site, as well as within the site. It includes security and communication arrangements. Staff entry, preparations to transition from outside to clinical work environment, including pause areas for relief. Emergency services, visitors. Goods, supplies and storage and waste removal and/ or treatment.
Support services key to the provision of clinical services should be separated so that the risks associated with that particular activity can be managed.
Support services are:
- Laboratory services
- Catering
- Laundry
- Environmental cleaning and housekeeping
- CSSD
- Maintenance and cleaning of surrounds, eg. waste areas
- Porters/”runners”, stretchers/wheelchairs
- Mortuary
- Security
Support services can be provided off-site, in which case, safe, secure and efficient transfer and logistical arrangements should be designed.
Auxiliary services: Auxiliary services are services which may be provided on or near the ACS site, but which are not directly related to core clinical care. This included overnight accommodation for staff who may not wish to return home to avoid exposing their families, or who need rest between shifts, or for discharged patients awaiting transport home, volunteers who have recovered from SARS-CoV-2.
Limited psychosocial services and allied health services may also be provided on or near ACS for example by approved partners.
Examples of schedules of accommodation for patient and support spaces for a protective isolation ward is available here and mild to severe cases here.
Clinical Services
Triage
Confirmed COVID-19 cases and PUIs who are referred from a testing facility or a higher level of care, will enter the facility in a triage area to receive vital screening and initial assessment. They will be registered and admitted to inpatient care. They will be assigned a "ward" or section of the facility - based on disease status and acuity. These are Protective Isolation, the Mild & Moderate ward, or the Critical & Severe wards. Patients should be clustered according to gender. As far as practicable, ablutions for each gender, isolation patients, paediatrics and staff shall be separately provided. Paediatrics patients, if admitted, are to be assigned a dedicated section. As patients recover or deteriorate, they may be relocated to the appropriate section/ ward. Once the patient has sufficiently recovered and a negative test result is received they will be appropriately decontaminated and discharged, collecting medication from the dispensary on exit. Patient movement between various sections of the ACS will be restricted as far as possible, with mobile radiology units, in-ward medication dispensing and in-ward food service.
Inpatient ACS accommodation
Separate spaces for:
- suspected, unconfirmed cases, under observation (PUIs), to be accommodated in isolation facilities (separate rooms, if possible);
- patients with confirmed COVID-19 with mild to moderate disease, not requiring dedicated oxygen therapy;
- patients who require dedicated oxygen therapy;
- patients requiring mechanical ventilation; and
- recovered/ confirmed negative.
Protective Isolation Facilities
Suspected, unconfirmed cases, under observation – persons under investigation (PUIs) to be accommodated in protective isolation facilities (separate positive-pressure rooms, if possible). PUI are restricted to their rooms. All food and laundry services will be brought to the PUI rooms to reduce interaction and potential contamination. All waste will be collected by facility staff and taken to waste handling areas. Infection prevention and control measures are put in place for the handling of used food utensil and laundry as well as waste collection. PUI areas will have restricted access, including for staff serving other inpatient sections, for confirmed cases.
Inpatients accommodation for confirmed COVID-19
Inpatient facilities confirmed positive COVID-19 can be accommodated in large shared ‘wards’ with partitioning between patients. Partitioning between patients is optional. Partitioning is preferable to curtains found in conventional hospitals, as they are more conducive to daily cleaning. If curtains are used, antimicrobial treated fabrics with biocide used to treat the curtains has been tested to international standard EN 14476 and shown to be effective against H1N1 Influenza A Virus (Swine Flu), >99.999% kill rate in 5 minutes and measles morbillivirus, >99.99% kill rate in 5 minutes are preferred. If only separate rooms are available, patient monitoring and surveillance will need to be accommodated. This phase of treatment has a lower area/space requirement compared with PUIs, as cross-infection between patients is less of a concern. Shared ablution facilities are acceptable. It is recommended that, at least, two general accommodation ward areas be provided.
a) Mild and moderate patients, and
b) Serious and critical patients.
The room must have openable windows for natural ventilation if a dedicated positive pressure ventilation system is not available. Ducted ventilation systems shall not be shared between PUI areas and confirmed COVID-19 patient areas.
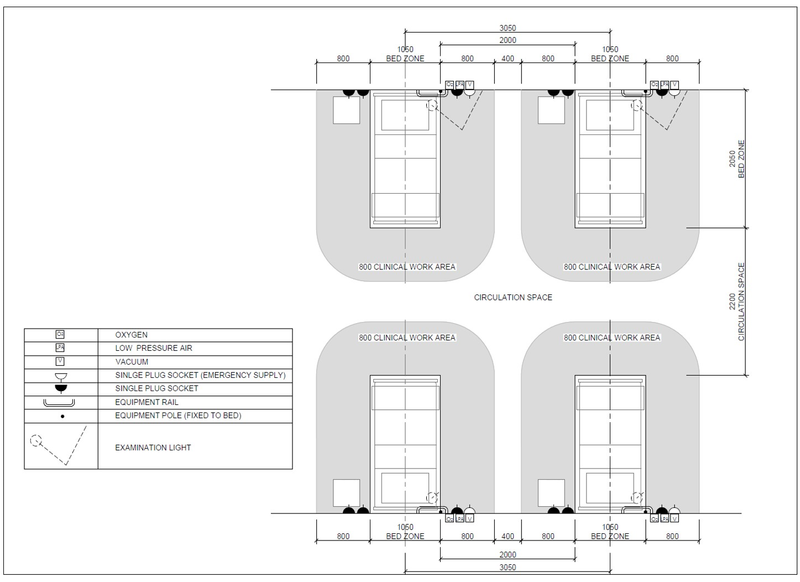
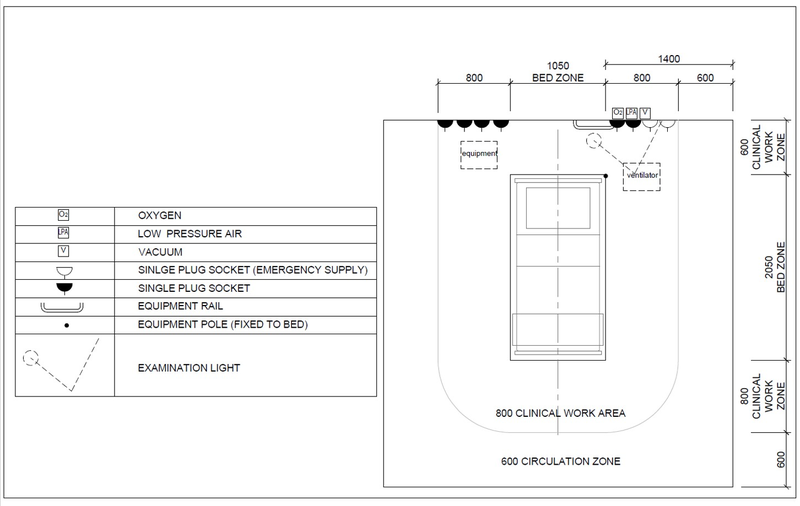
Examples above show bed layout with bed spacing for protective isolation, a mild/moderate patient and a mild/moderate patient shared ward and for a severe/critical patient
Patient services
Patients in ACS will not generally be ambulatory and will be confined to their room, or cubicle in a bed. In general, domestic beds or hospitality industry (hotel) beds are not suitable for patient care. These should only be used where a hospital-grade bed cannot be sourced, as hospital beds are designed for ease of cleaning and decontamination (for infection prevention and control) and with patient and ergonomics, safety and comfort taken into account (they prevent back injury for nursing staff and can help to prevent bedsores). The higher the specification of bed, the more suitable it is for the higher levels of care. Lockers for patients personal belongings should be provided in the uncontaminated zone (in which case bedside lockers will not be necessary), and it is preferable (where bedside lockers are not provided) for overbed tables to be provided, per bed, if possible.
The following beds are suitable:
- Repaired and refurbished beds from condemned hospital stocks.
- South African National Standard, SANS 521:2013 Edition 3.5, on Hospital beds and cots ISBN 978-0-626-28830-3.
- Beds listed on the National Treasury (See specification).
The table below details the minimum services required at each patient bed. Details on these services are discussed in a subsequent section of the document.
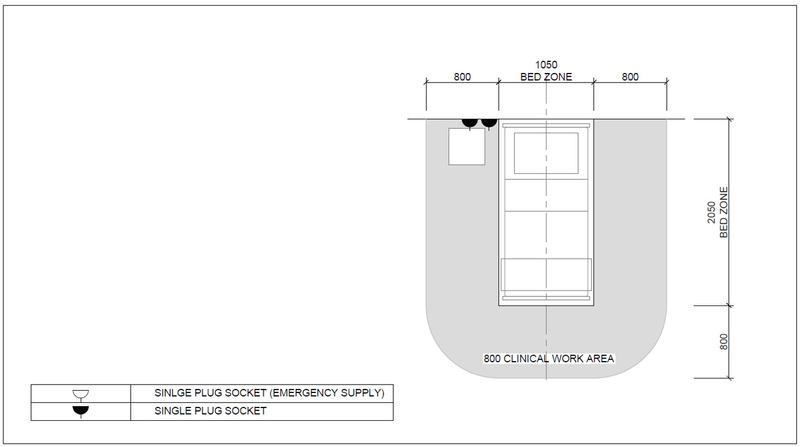
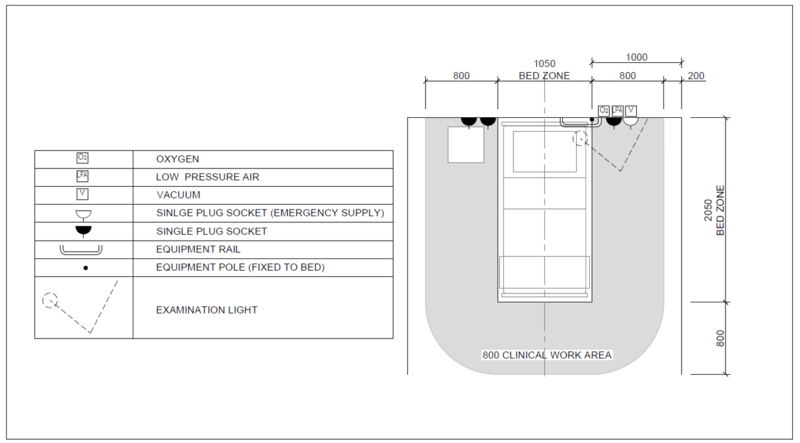
| Service/ Capacity | Triage | Isolation | Mild – moderate inpatient | Severe case wards | Critical case wards |
|---|---|---|---|---|---|
| Power – 16A 230V Single socket outlet | As needed | 1 per bed | 1 per bed | 3 per bed | 6 per bed |
| UPS Power – 16A 230V Single socket outlet | As needed | 1 per bed | 1 per bed | 1 per bed | 2 per bed |
| Medical Air[Note 1][Note 2] (LP)400kPa | No | Yes | No | Yes | Yes |
| Medical O2 -400kPa | Portable/shared | Portable/shared | No | One | Two |
| Vacuum-40kPa | No | Portable/shared | Portable/shared | Yes | Yes |
| Equipment rail | Yes | Yes | |||
| Upper room UVGI | Optional | Optional | Optional | ||
| Examination light | No | No | Yes | Yes | Yes |
| Room Ventilation rate | 60 L/s per person | 10 L/s per person | 10 L/s per person | 10 L/s per person | 12 ACH |
Notes:
- ↑ Mobile units recommended for intermittent use. 3 per 20 beds
- ↑ There are some ventilators which have built-in compressors allowing them to function without Medical Air. This is however, not the norm. With Ventilators probably being the most difficult medical device to obtain at present, it would be prudent to rather allow for Medical Air at each bed.
Two additional 16A 230V single socket outlets, one two-pin outlet and a worktop should be provided for every 32 beds (or part thereof), for:
- Electrocardiograph (ECG): Could be omitted if monitors have a full 12 lead ECG function.
- Blood gas analyser: Could be omitted if a Lab Services are available.
- Staff cellphone charging.
Example of healthcare technology to be provided for critical care patients is shown in this schedule. Severe patients may be provided continuous positive airway pressure (CPAP). Emergency trolleys (“crash carts”) are to be provided in patient areas with convenient access to patient beds, out of the passage of corridors and is moved to the patient when needed. 1 crash cart for every 16 patients (or part thereof, with at least one dedicated for PUIs. An example of provisions for a crash cart is shown in this schedule
Patient ablutions
SARS-CoV-2 is found in faecal matter, so careful management of patient body fluids is crucial and convenient, practical support for frequent cleaning of ablutions especially shared ablutions is necessary. Dedicated ablutions (toilets and showers) are to be provided for patient use. Toilets and showers should be in separate rooms. Hand washbasins and or/ hand sanitiser should be provided both inside and outside the toilet room so that patients can wash their hands on the way in and on the way out of the room.
- 1 toilet for every 8 persons.
- 1 shower for every 8 persons.
- 1 disabled ablution for every 8 regular ablutions (or part thereof).
- 1 disabled shower for every 8 persons (or part thereof).
Critical and severe patients may be sedated and have a reduced need to access ablutions, ablution facilities proximity and provision can take this factor into account. Showers and wash hand basins should have hot and cold running water. Where possible ablution facilities must have openable windows for natural ventilation, if not possible the bathroom extraction and room ventilation system must be reviewed before admitting patient (see ventilation).
Portable toilets and showers may be used, provided that suitable hand wash facilities are provided. These will need to be suitably located, preferably in decentralised clusters, so that patients can easily access them without needing to walk very far. Ablutions should be located and designed in such a way as to provide visual and acoustic privacy, dignity and avoid disturbance of other nearby patients when accessing, using or cleaning the ablutions. Separate ablutions are to be provided for PUIs and confirmed patients.
Makeshift sluice areas
In conventional hospital settings, sluice rooms are provided for cleaning and sanitation of soiled equipment, such as bedpans. In a temporary setting, such as an ACS, the establishment of a temporary sluice room may not be practicable, and there may not be facilities for emptying buckets, rinsing equipment etc.. The following is suggested: Allocate a toilet, hand wash basin, not in splash range and restrict access to it for draining buckets and install a macerator for disposal of disposable bedpans. Electrical, water and waste supply points required as per supplier specification.
Dedicated patient treatment areas
The following dedicated, private spaces per ward for clinical procedures are recommended:
- Counselling and consulting room (can be shared), as shown in the figure below
- Minor procedures room, as per the example provided in the figure below
Logistical Services
Communications
Electronic communication should be facilitated in all zones of the ACS by the provision of device charging stations, and wifi.
Visitors entry point
Visitors are strongly discouraged from entering the ACS.
- In paediatric wards, one parent may be accommodated to visit a patient. In such cases, direct access for the visitor should be provided so that the visitor does not need to pass through the general patient area. Appropriate PPE must be donned before entering the patient area and hand washing/sanitising must be done when exiting the area.
- Non-patients who are accompanying suspected patients to the facility for testing or admission must be accommodated in a well-ventilated, spacious waiting area. Signage in such waiting areas must inform visitors about symptoms, hand hygiene and PPE.
- Hand washing/sanitizing facilities should be provided.
Staff areas
Staff change rooms
A minimum of 9m² or 4m² for a single person, increasing by one m² for each additional person is required. The clean (street side) and dirty (contaminated facility side) of the change room should be separated by a step-over barrier.
Staff rest areas
Staff rest areas within the main facility should be provided with access to kitchenette facilities and staff ablutions.
Staff auxiliary services
Staff on-call:Since staff may be required to work long hours or could be required to be on-call, shared, overnight sleeping facilities can be provided for staff on duty, outside the contaminated area, but in close proximity on the patient areas. An example is set out in the diagram below.
Staff accommodation: It is advisable to provide some staff accommodation for off-duty staff who may not have suitable alternatives, for example homes too far away, transport limitations or the requirement not to expose family members to risk. This should be provided in the vicinity of the ACS, but in a physically separated zone. This amenity can be outsourced.
Support Services
Workflow principle
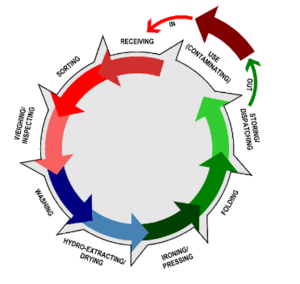
Progressive workflow from “dirty” (that is contaminated) to “clean” linen is advisable to reduce the risk of exposure to contaminated materials. The workflow diagram below, showing the progression from the dirty linen receiving area, to the cleaning process, to decontamination and drying, and finally sorting and packing, and storage, illustrates this principle.
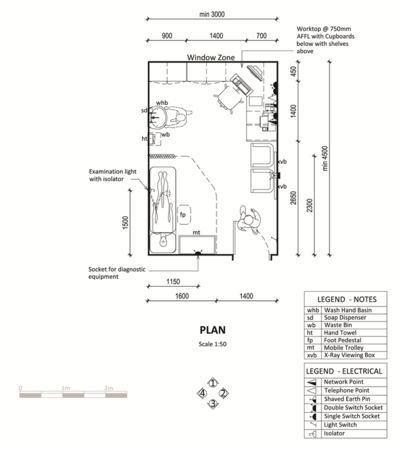
Laboratory
The WHO recommends the following laboratory diagnostic equipment be accommodated:[2].
• Lab screening test kit • Lab confirmation test kit • RT-PCR kit • Extraction kit • Cartridges for RT-PCR automatic systems • Swab and Viral transport medium
Additional accommodation requirements are:
- Reception counter- receiving specimens
- Testing with perspex/ glass screen
- Receiving/Data capture
- Specimen holding
- Toilet - staff
- Blood storage fridge
Can be provided as a modular laboratory unit as shown in the diagram.
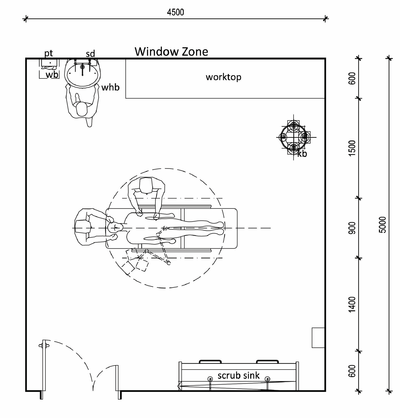
Pharmacy
The purpose of the pharmacy is to provide medicines needed for inpatient treatment and care. All medical supplies should be stored in a secure, climate-controlled area in close proximity to the patient treatment area. The pharmacy must have dry, lockable, climate-controlled storage of medications. Most pharmaceuticals are labelled with storage temperatures. The pharmacy should have adequate ventilation through an openable window to prevent humidity from building up in the room. Air-conditioning or mechanical ventilation can be provided, if necessary.
Dispensing areas must be well lit. Worktop in space for stock records and administration. Dispensing counters to have perspex or glass screens to serveries. Social distancing implemented at counter, between pharmacists/assistants and between chairs in waiting area.
Can be provided in a mobile unit.
Radiology
The purpose of radiology services is to provide chest X-Ray services for COVID-19 diagnostics. In general, CT scans, bucky rooms etc. associated with some radiology equipment require specialised infrastructure and therefore is not suitable for ACS. Radiology services can be provided as a mobile floor standing unit, or containerised unit. Alternative technologies such as Lodox and hand-held ultrasound devices are being investigated as potential options and could be confirmed as suitable for use in due course.
Laundry services
All dirty linen should be handled for bagging or binning inside the patient room/cohort area. The clean linen stock should be stored conveniently close to clinical areas, in a dedicated clean area in the uncontaminated zone. Used linen should be stored in a designated, safe, lockable holding area while awaiting collection. Interim storage areas for soiled linen at the wards is allowable; this may be in dirty linen/ utility room. Any clean linen for PUI areas should be handled in spaces physically separate from dirty linen of confirmed patient areas. It may be necessary to completely separate PUI and confirmed patient linen streams. Soiled linen and clean linen bags and bins should be dedicated and not mixed. Full laundry cleaning and drying services may be provided on-site or outsourced. If laundry cleaning and drying services were already rendered on or for the host site before it is repurposed as an ACS, then a suitability and risk assessment should be conducted to ensure that the volumes of laundry generated and infection prevention and control measures are conducive and modifications made as necessary. A new full laundry service may take time and resources to establish, and in general, will not be established at a host site as a temporary solution. Where the site and circumstances advocate for the design of a new laundry or the upgrade of an existing laundry, the IUSS Laundry Services for Hospitals should be applied.
Siting and model selection considerations
When an existing laundry is being assessed for use or a new one is being planned the following considerations apply:
- Water and power capacity.
- Ease of access to the ACS’s main corridors and internal transport routes.
- The noise factor of the facility and its impact on nearby patient care departments.
For outsourced departments:
- Delivery areas to allow sufficient space to ensure that vehicles can manoeuvre and park easily at the reception and dispatch bays.
- Access to the ACS service roads and public roads.
Functional requirements
The most basic equipment needed in a laundry includes washing machines, tumble dryers and ironing machines. Equipment requiring steam is not recommended for a temporary facility. The sizing of the laundry, equipment and engineering services can be modified based on the principles provided in the IUSS Laundry and linen.
Catering services
Kitchenettes, that is, areas for tea, coffee and snacks, mainly for staff, in staff pause areas are discussed elsewhere in this document. Catering services (for staff and patients) may be provided on- or off-site. If the ACS is to be established with easy access to a suitable, existing, functional kitchen service (e.g. hotel, military or hospital catering) which can meet the additional demand of the ACS, then this should be used. If there is no suitable facility, catering should be outsourced via a suitable off-site supplier. Only in the event that no feasible or suitable, existing facility or local supplier is available, should a new catering service be established at the ACS. Detailed guidance for the sizing, design and layout of catering services can be found in the IUSS Catering Services for Hospitals. The kitchen (for on-site catering) or preparation area (for off-site catering) should be located with easy access to the point of delivery and storage of food. Adequate food and equipment storage space must be provided. It is recommended that patient and staff meals, where provided, be supplied in disposable, containers, suitable for incineration, and that these are disposed of as risk waste immediately after use. Where off-site catering is used, a suitable area for receiving should be provided. Space will be required for sorting meals for distribution and collecting and storing dirty dishes, washing dirty dishes, if necessary, and disposing of left-over food and disposable containers and utensils. The size of the areas required for this will depend on the number of meals delivered.
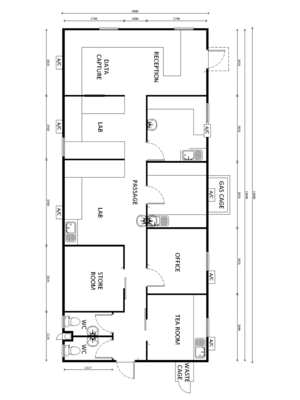
CSSD
The primary function of a Central Sterile Supply Department (CSSD) is to provide an efficient, economic, continuous and quality supply of disinfected and sterilised items, when needed, to all patient-care service points in the ACS, and to receive returned contaminated items for cleaning. CSSD with limited sterilisation capacity (autoclave) but sufficient disinfection capacity (instrument washing). The layout requires a designated clean and dirty areas with a hard barrier between to avoid cross-over of staff and equipment. Work is unidirectional - flows from dirty – to clean - to sterile areas. This yields three distinct zones: 1. Receiving and cleaning with pass-through windows
- Dirty receiving with Perspex or glass partitioning
- Dirty utility
- Decontamination and cleaning/wash area with throughput instrument washing
- Trolley wash/Park – external
2. Inspection, assembly and packaging with pass-through windows
- A tabletop autoclave
- Instrument washer
- Clean Packing area
3. Sterile processing, storage and distribution. (Separate issuing area from receiving area) with pass-through windows
- Store -linen and consumables
- Sterile pack store
- Issue - Collection hatch with Perspex or glass partitioning
Must also have a secure, separate receiving area for consumables receiving and storage.
An example of a CSSD unit is shown in Figure 21.T
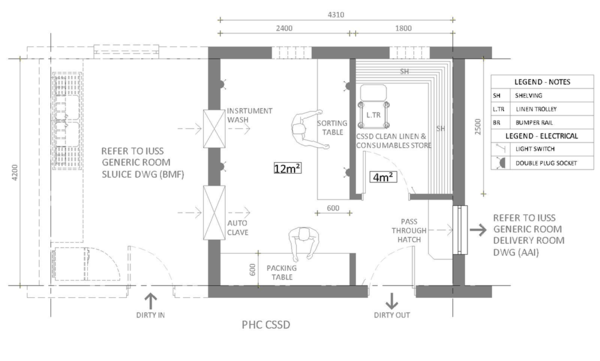
To be understood: Quality of services, eg. water (including a source of distilled or ionised water for rinsing of bronchoscopes) and electricity, quality of management, if equipment is appropriate for needs and fit for purpose, what is required of the equipment in the near and far future (relocation?), compliance with S.A. National Standards for CSSD, compatibility of equipment and devices, correct chemicals, maintenance of equipment, training of operators.Although the use of disposable breathing circuits and accessories (masks) should be encouraged, the capacity to disinfect these items if disposable is not available must be considered.
he CSSD must be sized to serve the ACS.
Workload: Number and type of procedures, number of procedure rooms, eg for bronchoscopy, operating hours, available inventory, volume of work and peak times, degree of mechanization, eg. manual or automated instrument washing, amount of product to be stored – chemicals, barrier wrap, chemical indicators, etc., storage and distribution records, quality records – eg. equipment checks, disposal needs.
Space requirements: Space for separation of clean and dirty, allow for unidirectional movement, allow space to manoeuvre trolleys (queue, pack/unload), reduce lifting and carrying heavy items, reduce awkward movements, allow for tidy work areas. Insufficient space will compromise sterility!
Preference should be given to construction materials and finishes which are suitable for frequent cleaning and tolerant to chemicals, including bleach (sodium hypochlorite). Impermeable flooring, non-slip and smooth washable walls are needed. Joints at walls and floors and coving at wall edge, and exposed drains should be avoided. Worktops should be sealed and should be ergonomically suitable.
Below ceiling height of at least 2.8m. Ceiling compliant with ISO 14644-5:2004 – resistant to humidity where steam and moisture are present. Noise – insulation of washer-disinfector and steriliser in technical walls will reduce noise Lighting: Natural light if possible – windows sealed. High luminance if artificial lighting. Open, slatted shelves for sterile store area. Sinks for manual washing: At least two basins, deep (25cm at least) basins, 91 cm from floor, wide and long enough for the biggest instrument tray or container, water ports for flushing of lumens.
The CSSD space allocation and layout should be determine based on what procedures and which medical devices will be required. A typical equipment list is provided here.
Procedure Manual – see CFSA SOPs. Include: Sharps injury SOP Waste removal SOP
References: IUSS documents CFSA: CSSD Forums of South Africa – documents and training course SANS
Maintenance and cleaning
Maintenance and cleaning services must be accommodated with offices located away from clinical areas.
Mortuary services
The National Department of Health has issued guidance on handling of dead bodies and infectious remains[4], which should be applied to ACS. While some guidelines have recommend that bodies of persons who have died from COVID-19 should only be held for a very brief period prior to cremation or treatment for burial[5], the WHO holds the view (at the time of writing) that there is no evidence of persons becoming infected from exposure to bodies during normal ceremonial and burial activities. However, appropriate PPE should be used when handling such bodies with additional airborne precautions to be taken during autopsies[6].
Either body cabinets or a refrigerated room could be used for body storage.
Location and layout of mortuary service
It is likely that not all alternative care sites will have a mortuary. Those without a mortuary must have a holding room that is located away from general access areas. This holding room must be suitably sized and conditioned. A recommended room size is 3.5 m x 3.4 m[7] A mortuary should be located so that it is easily accessible to mortuary staff and related service providers and visitors without presenting either aesthetic, emotional or ethical problems for unrelated staff, patients or visitors. It should be separate from the general facility, allowing access for the family to view a body without passing through any potentially contaminated area of the facility. The visitors’ entrance should be external and completely separate from other access points. Appropriate routes should be designated so that bodies are not moved through public-access areas.
Sizing of mortuary
The layout and size of a mortuary are largely determined by the number of bodies stored and whether body storage needs to be in cabinets or in refrigerated rooms.
Services
The following services are required in a mortuary:
- Hygienic floor drains that are resistant to corrosion from blood and chlorine should be provided in all “wet areas” of the mortuary and should be directly connected to the sewer system. These areas include body preparation, autopsy space, etc. These areas require thorough cleaning after every procedure, using large quantities of water and decontaminating and disinfecting chemicals and soaps.
- Sluicing facilities are to be provided in both the body-preparation and autopsy areas if they are not a common area.
- Open floor channels should be avoided. Where this is not possible, these should be covered by durable, flush-fitted stainless steel grids.
- No sewer connections external to the mortuary services should be made to the line between the wet area drains and the main sewer system in order to prevent backflow to other areas.
- The provision of hot and cold water in the facility is imperative, with all basins, sinks, ablution areas and autopsy tables being provided with both.
- Anti-backflow devices should be fitted to the water-supply lines serving mortuary table faucets to prevent backflow should supply water pressure fail.
- Electricity supply to the mortuary – particularly for refrigeration purposes – is to be provided from the essential supply system for the hospital. Alternatively, a back-up generator is to be supplied to allow for the maintenance of required temperatures in the cooling/freezing facilities in the mortuary.
Finishes
Wall and floor finishes should be impervious to liquids and easily cleanable.
Notes and References:
- ↑ Test suspect COVID-19 cases according to diagnostic strategy
- ↑ IUSS 2014 https://www.iussonline.co.za/norms-standards/support-services/30-laundry-and-linen-department
- ↑ WHO 2020, https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/covid-19-critical-items
- ↑ IUSS 2014, https://www.iussonline.co.za/norms-standards/support-services/24-central-sterile-service-department
- ↑ National Department of Health South Africa, 2020 https://www.nicd.ac.za/wp-content/uploads/2020/03/COVID-19-ENVIRONMENTAL-HEALTH-GUIDELINE-1.pdf
- ↑ Zhejiang University, 2020 http://www.zju.edu.cn/english/2020/0323/c19573a1987520/page.htm
- ↑ WHO, 24 March 2020 https://apps.who.int/iris/bitstream/handle/10665/331538/WHO-COVID-19-lPC_DBMgmt-2020.1-eng.pdf
- ↑ IUSS Adult Inpatient Services, 2014, [3]
Environmental Controls
General Indoor Environment Conditions
Existing environmental control systems should be modified to suit requirements in the facility. The following issues should be considered:
- Systems should be set to maximise the introduction of fresh air and maintain the pressure regime (see ventilation).
- The following internal temperature range should be maintained 19 - 24oC.
- Cooling systems should be able to cater for projected internal heat gains from people, lighting and equipment. Indicative heat gains in treatment areas are 8W/m2 from people, 15W/m2 from lighting and 3 W/m2 equipment and in critical care areas 16W/m2 from people, 15W/m2 from lighting and 60W/m2 equipment.
- As heat gain can vary widely between items of equipment, heat gain and utilisation rates for equipment should be obtained from the manufacturer to establish this more accurately.
Solid waste from ACS
According to the National Department of Health COVID-19 Environmental Health Guidelines “All solid waste from the facility should be regarded as potentially infectious material and therefore appropriate precautions should be taken". The management of healthcare risk waste (HCRW) should follow the correct identification segregation, storage and disposal processes as indicated in SANS 10248-1.
- HCRW is segregated at the point of generation and shall be containerized to minimize the risk of contamination.
- Waste generated from patients in isolation or quarantine in a designated facility health facility is treated as health care risk waste (HCRW) as per SANS 10248-1-2008.
- The HCRW is properly packaged in a sealed, leak and puncture-proof containers/ boxes.
- The HCRW is labelled with the biohazard symbol/ sign and marked “Corona virus or COVID-19”.
- The HCRW is stored separately from other waste generated.
- The collection, transportation, treatment and disposal is provided by only an appointed/ appropriate contractor/ service provider, however, ensure that waste is safely stored until the health care waste management company can pick it up and that the company knows and acknowledges that waste was generated by suspected or confirmed COVID-19.
- The waste management company collecting must ensure that and treated and disposal is conducted at license waste treatment/ disposal facilities .
- All personnel or staff in contact with patients must be geared with appreciate personal protective equipment (PPE’s) at all times to prevent exposure or risk to health.
- Monitoring should be done at such facilities.
- All, bags, bins and boxes must be adequately sealed, as not to leak any fluids, and must be wiped down with 0.05% chlorine solution
Measures developed should consider the following.
- Develop a waste management plan following national guidelines and best practice standards for the disposal of medical waste (WHO, 2020).
- Establish procedures with medical waste service providers to regularly pick up the waste and dispose of this safely.
- Provision should be made for 5kg of solid waste per bed per day and this should be monitored and supplemented where it appears this may be inadequate.
- Ensure that access to waste is secure and controlled, for instance, by using lockable waste 1000l containers kept in a location that can only be accessed by health facility and nominated service delivery staff.
- Vermin control programs must be implemented throughout the site with HCRW collection points prioritised
- Provision for safe cleaning and disinfection of reusable containers should be provided. Cardboard, single-use HCRW boxes are removed from site and incinerated.
- Waste must not be allowed to accumulate or be stored inappropriate or unsecured containers.
Engineering Services
Engineering services include patient services, ventilation, electrical power, water, medical gases, oxygen, compressed air, vacuum, lighting, and fire safety that support the needs of the patients and medical staff under normal and emergency situations. Good practice standards are provided in:
The guidance below draws on these and other manuals and standards.
Building ventilation
Mechanical ventilation
While SARS-CoV-2 RNA has been detected in aerosol form, in experimental mechanical aerosolization studies, it is primarily spread through droplet and contact spread and the potential for airborne transmission is thought to be low. However, the following advice is provided by ASHRAE regarding HVAC systems in general spaces (not specific to healthcare): Effective high levels of ventilation must be achieved in the facility. Existing ventilation systems should be tailored to suit internal layouts and requirements and the following measures should be taken.
- Mechanical systems should be set to maximise fresh air supply to the facility. There should be no recirculated air without HEPA filtration or other validated decontamination processes.
- A pressure regime should be established, as shown in figure 2, to 'push' air from clean areas, to dirty areas and then out of the building.
- A clean air supply of over 10 L/s per person should be targeted for odour control.
- Fresh air supply shall not be located near patient beds to avoid drafts in winter.
- Extraction points can be located near patient beds in isolation wards or at a high level in long-stay wards. Short-circuiting of air between high-level supply and extraction is a performance risk in winter.
- Noise from ventilation systems and fans shall be below 45 dBA
- Protected lobbies, internal partitions, door arrangements, fans and extracts should be used to maintain the pressure regime and airflow as indicated in the diagram below.
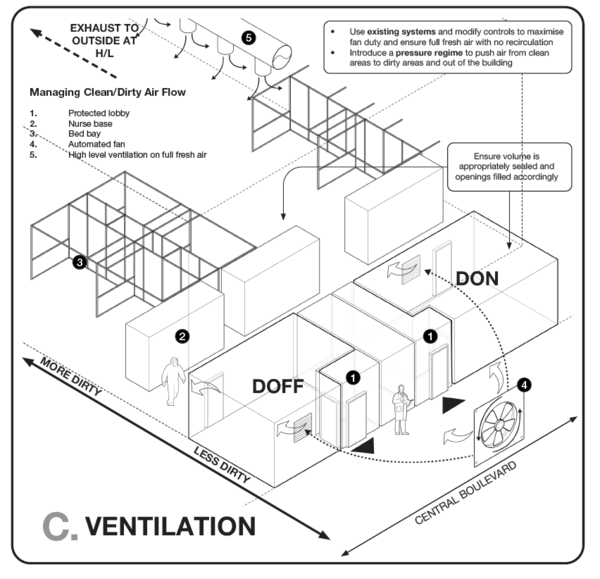
Natural ventilation
The airflow direction between naturally ventilated zones may fluctuate according to the wind direction. This is not of concern within and between contaminated zones where occupants are either protected with mandatory PPE or confirmed to be infected with disease strains similar enough so as not be able to reinfect others. Where levels of occupant susceptibility to an airborne disease is significantly different, the less susceptible areas (such as PUI areas and uncontaminated areas) shall be protected from the naturally ventilated and contaminated areas by mechanical ventilation systems with a capacity sufficient to overcome the expected natural ventilation pressure fluctuations (typically 5-15Pa). Where such mechanical ventilation systems are not feasible or expected wind pressure is too great, zone adjacently must be avoided.
Ultraviolet germicidal Irradiation
The application of Ultraviolet Germicidal Irradiation (UVGI) for room air disinfection is well understood and is proven to be effective in the disinfection of microorganisms including M. tuberculosis (TB) in air. UVGI should, therefore, be considered as a valid element in indoor airborne infection control strategy for high volume settings. Studies have demonstrated the importance of good vertical air mixing in the room, and the safety of UVGI application. In areas where UVGI is indicated, the design and development of UVGI systems should be in accordance with the Abridged UVGI guide[3]
Electrical power
Sufficient and reliable power must be available at the facility for envisaged medical equipment, medical gases, lighting and building ventilation equipment. Power installations for the temporary facility can be divided into three zones as indicated below. These are existing services, the temporary service zone and services in each bay. The following should be considered by competent engineering professionals.
Existing services
- Capacity: Evaluate whether sufficient power to accommodate envisaged medical equipment, additional lighting and heating, building ventilation and air conditioning can be provided. If existing capacity is insufficient, investigate if it is possible to route additional power from additional locations/transformers around the site or from adjacent sites.
- Safety: The existing electrical distribution network must be able to supply the required equipment load. If this is insufficient/appears unreliable, identify how this can be supported.
- Resilience: Evaluate back-up power and a UPS capacity against essential services demand. If existing capacity is not sufficient, source and establish temporary service capacity.
Temporary service zones
- Identify locations for temporary service zones where equipment can be located.
- Ensure that equipment and maintenance access is safe and easy.
- Ensure that all distribution boards, circuit breakers and cables are clearly labelled.
Services in each bay
- Provide pre-wired power strips/trunking as per bay requirements.
- Check that these include sufficient electrical outlets and service points for envisaged equipment.
- Ensure that trunking will carry required equipment loadings. The IUSS Building Engineering Services Guide can be used to check requirements[1].
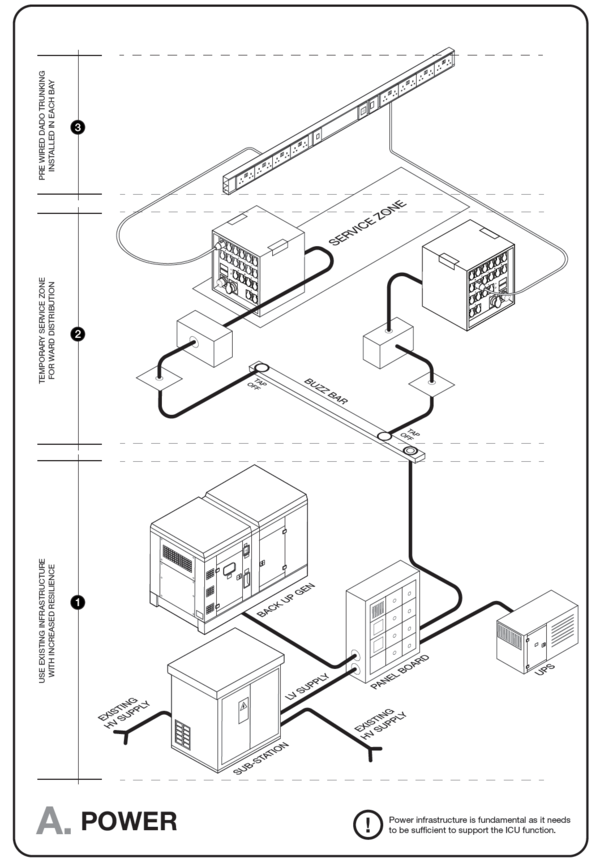
Water
Water points are needed for handwashing, showers and cleaning. The following issues need to be taken into account.
Supply
Onsite cold water storage, dedicated to the domestic water requirements of the facility, should be provided. A minimum usable volume of 500 litres per bed should be available. Hot water storage and consumption should be confirmed by an engineer, as follows:
- Storage 25 L per bed.
- Consumption 180 L/bed.day W/O laundry; 250L /bed. day W laundry.
Handwashing
See infection control for clinical wash-hand basins
Showers
Showers for staff coming off shift should be available. Staff flow routes exiting contaminated treatment areas should pass through gowning and shower areas.
Medical gases, oxygen and vacuum (suction)
Medical gases, oxygen and vacuum services will be required in the facility. Mobile gas supply requires piping between supply and patient and clutter floor space. Preferably used fixed installations for patient rooms/cubicles, if possible.
System capacity and point of use pressures and consumption rates are to be ensured at all points. WHO provides technical guidance on oxygen sources and distribution.
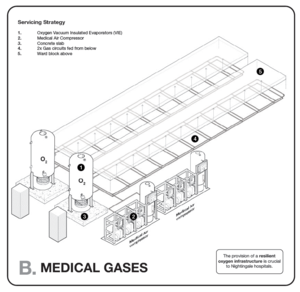
The following points should also be taken into account.
- For centrally supplied medical gas and vacuum services, system resilience and availability must be ensured.
- Compressors, tanks, accumulators, VIEs, headers and controllers must be secured from uncontrolled access.
- Where possible, gas piping should be reticulated below floors or in ceilings to ensure protection from tampering, damage and ease of access.
- Where reticulation is within open areas, high-level reticulation with point-of-use droppers is advised. Low-level reticulation within rooms is to be avoided.
- Flexible piping can be used, ensuring it does not present a contamination or fire risk and should comply with local regulations. Special care should be taken with flexible O2 piping, keeping it to a minimum.
- Vacuum piping may be contaminated, where point-of-use filtration and collection systems fail. Precautions should be taken when demounting or disconnecting temporary vacuum lines.
- Ensure that oxygen pipelines are designed to provide sufficient flow to all oxygen points in the facility. In terms of utilities, oxygen and medical air would be required. A temporary vacuum point can be provided by mobile medical vacuum units distributed throughout the unit.
- Mobile medical air supplies should be considered where reaching piped specifications are not feasible. Where flexible hoses are used for oxygen and medical air special precautions need to be taken. Flexible oxygen piping must be chemically safe for O2 use. Where perishable flexible piping is used for medical air, terminal filtration at the point-of-use may be required at point-of-use. Especially for long-term use.
- Electrical and gas services can be reticulated against pipe racks or boards fixed to the bed-heads for head to head bed arrangements.
- Gas service isolation valves should be carefully positioned for each clinical unit to avoid shutdowns of major sections.
- Gas service outlets to be labelled and colour-coded with 3mm lettering.
- SANS 7396-1 should be used to specify the requirements from design to commissioning of medical gas and vacuum systems.
- Medical gas and vacuum pipelines shall be marked per SANS 7396-1 and ISO 5359, as applicable.
- Colour coding of non-medical gas piping must be as per SANS 10140-3:2003.
- SANS 1409, as amended, specifies the requirements for non-interchangeable outlet sockets and probes for specific medical (gas and vacuum) services used in hospitals.
- Plain-ended copper tubing for low-pressure medical gas and vacuum shall comply with the requirements of SANS 1453 and SANS 1067-1 or SANS 1067-2, as deemed suitable.
- Laboratory gas taps and valves shall be marked as described in SANS 10140-4.
Lighting
Existing lighting systems may need to be modified to suit the clinical requirements in the facility. High bay lighting presents inadequate colour rendering quality for the accurate detection or easy diagnosis of certain clinical conditions. This needs to be evaluated in the selection of supplementary lighting systems.
- Lighting levels should be provided in line with the indoor lighting levels recommended in the Table 6 of IUSS Building Engineering Services .
- Mobile task lighting systems may be adopted in the serious and critical stay wards to supplement incorrect lighting quality.
- Emergency lighting and illuminated emergency egress signage should be linked to the back-up power system.
- External security lighting in external parking areas and spaces around the building should be enhanced to ensure the security of medical staff who need to change shifts at night.
Fire safety
A functional fire alarm system should be available to support the patient care setting. Fire is a very real threat due to the possibility of an oxygen-enriched atmosphere developing so ventilation is crucial. The use of temporary facilities for medical care should note the following fire risks (NHS, 2020):
- Patients may have a very high dependency.
- Areas are not specifically designed for patients and do not meet guidance on fire compartmentation and progressive horizontal evacuation.
- Large numbers of patients supplied with oxygen up to 10 litres per minute.
- Possibility of oxygen concentrations exceeding those generally found in the atmosphere- less risk if effective ventilation or large volume i.e. high ceilings.
- Possibility of storage, in excess of 40 litres, of alcohol-based chemicals (such as hand-rub), necessitating a flammables cabinet on site.
- Staff who may not normally work together .
- Staff who may not be familiar with the area.
- Staff not trained in fire safety, progressive horizontal evacuation or oxygen isolation for the specific area.
These factors should be taken into account in fire risk assessments which should then address significant findings in an action plan. Fire assessments should be undertaken by a qualified person and shared with operations and building management staff within the facility. Measures developed should include:
- An automatic fire detection system
- An emergency egress plans are prepared that include patients who have a very high dependency.
- Signage, notices and lighting are installed and are working effectively.
- Management processes are in place to minimise the risk of fire from ignition sources, fuels and oxygen.
- Staff are trained and a fire safety guide sheet for staff is developed and issued.
- Emergency egress routes are kept clear.
Notes and References:
- ↑ 1.0 1.1 IUSS 2017, https://www.iussonline.co.za/docman/document/healthcare-environment-crosscutting-issues/91-iuss-building-engineering-services-gazetted-1/file
- ↑ 2.0 2.1 2.2 2.3 BDP 2020, http://www.bdp.com/globalassets/projects/nhs-nightingale-hospital/nhs-nightingale-instruction-manual.pdf
- ↑ van Reenen et al,2019 Abridged UVGI guide
Additional Resources
A Directory of Service Providers can be viewed here
References