Building Engineering Services
This page or section needs to be cleaned up. |
Please help to expand this page. |
POLICY AND SERVICE CONTEXT
Overview
Many of the Building Engineering Services of a health facility have specialised needs within the context of healthcare provision and infection prevention and control. Specialist needs may include a combination of hygiene, redundancy and contamination-control requirements over and above the normal best engineering practice.
The Building Engineering Services dealt with in this document include: ventilation systems, wet services, gas and vacuum services, electrical services and electronic services. The primary function of this document is to provide terms of reference to designers who are contacted to develop building engineering services systems. This document does not serve as a principal facility planning guide but as a best-practice guide within any planned level of healthcare service.
“This document describes engineering design, installation and commissioning principles in terms of current specialist clinical, contamination control and maintenance requirements“
Policy and Service Context
Context
This document serves as guidance in the development of all levels of the healthcare facility. Certain sections may not be applicable to all considered levels of facility although, where a certain engineering service is supplied, that service shall be developed in accordance with the guiding principles contained herein.
Design principles
This document will detail design principles within the scope of services described in the Engineering Council of South Africa’s gazetted Guideline scope of services and tariff of fees in terms of the Engineering Professions Act (46 of 200). This document will also describe design, installation and commissioning principles in terms of current specialist clinical, contamination-control and maintenance requirements. While this document details design requirements and acceptance criteria which have an impact on clinical services, these requirements are prescribed within the framework of the entire IUSS set of guidance documents, and cannot be viewed in isolation. The following documents should be complied with, together with this document:
Within the South African healthcare context, many clinical and administrative zones may be subject to infection prevention and control measures with particular consideration for airborne contamination control.
| Clinical services | Essential | Recommended | Support Services | Essential | Recommended | Healthcare environment/
Crosscutting issues |
Essential | Recommended | Procurement &
Operation |
Essential | Recommended |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Impatient services | X | Administration & related services | Generic room data | X | Integrated infrastructure planning | X | |||||
| Laboratories | X | General hospital support services | Hospital design principles | X | Project planning & briefing | X | |||||
| Mental Health Services | Catering services | Engineering design principles | X | Space guidelines | |||||||
| Critical care | Laundry and Linen | Environment and sustainability | X | Cost Guidelines | X | ||||||
| Emergency centres | Mortuary | X | Materials & finishes | X | Procurement liaison | ||||||
| Obstetrics & gynaecology | Nursing colleges | Future healthcare environments | X | Commissioning | X | ||||||
| Oncology | Health facility residential | Healthcare technology | Maintenance | X | |||||||
| Outpatient services | X | Sterile supply | X | Inclusive environments | X | Decommissioning | X | ||||
| Paediatrics | Clinical training | Infection prevention & control | X | Capacity development | X | ||||||
| Pharmacy | Waste disposal | X | Health informatix | ||||||||
| Primary health care | Regulations | X | |||||||||
| Diagnostic radiology | X | ||||||||||
| Rehabilitation services | |||||||||||
| Sub-acute services | |||||||||||
| Surgery | X | ||||||||||
| TB | X |
Where this document lacks guidance on a topic or appears to contradict the requirements of the guidelines identified above, the guidance of those documents will take priority.
Service Context
Levels of care
- “Levels of Care” is discussed in detail in the Project Planning and Briefing document. The Building Engineering Services document does not prescribe levels of care within the healthcare system and does not delineate the application of technology within these levels. It intends only to describe the building engineering services and technical aspects that should be considered from the concept development to the closeout and handover stages of the project. It is not incumbent on the engineer to prescribe appropriate levels of care and this subject is therefore not addressed herein. The allocation of appropriate technologies and services within the prescribed levels of care is a function of the engineer during the facility-planning stage as described by this document.
- In this document, where three distinct options are made describing system quantities or capacities, these are to be interpreted as the minimum acceptable standard, recommended best practice, and maximum practical limit respectively. Where only two options are given, these are to be interpreted as the minimum standard and best practice respectively. Where only one option is given, this is to be interpreted as the minimum acceptable standard. The reader is cautioned not to interpret these capacity standards as levels of care.
-
King George V (KZN 2013)
PLANNING AND DESIGN
Overview
The national and provincial service and policy context should be the basic determinant of planning and design principles in the public sector
The national and provincial service and policy context (Part A of this document) is the basic determinant of planning and design principles in the public sector. In the private sector, planning and design will have determinants as defined by the service provider, within certain minimum prescribed limits. Part B describes the scope of planning and design guidance, design considerations and functional relationships between engineering systems. These principles are subsequently developed into a series of Design Specifications (Part C), Commissioning, Handover and Decommissioning (Part D) including some case studies (Part E). Parts C, D and E are intended to demonstrate how the principles prescribed in Part B should be applied. Parts C and D, if used directly, are deemed to satisfy the principles developed in Part B, but are not the only acceptable solutions. Case studies (Part E) are for illustrative purposes, to demonstrate worked solutions and should not be adopted without appropriate contextual adaptation
Stages of design and implementation
- It is critical that building engineering services professionals involve themselves in the early stages of a project’s initial planning, studies, investigations and assessments. Exclusion or late inclusion of an engineering team from the planning stages of a multi-disciplinary construction project presents a considerable risk of resulting, not in savings, but fruitless expenditure, design delays and ultimately compromises in the functional and build quality of the product.
- The scoping and broad coordination of services is invaluable during concept development, and the value-added through the early inclusion of building services professionals is frequently underestimated.
- Briefing authorities or developers are therefore encouraged to ensure that the client’s representative consults with a team of engineering professionals during the earliest project-planning stages. The deliverables of the concept and viability study stages should, therefore, include the following:
- Summaries of collated information
- Reports on technical feasibility, benefits and risks
- Reports on regulatory compliance issues
- Reports on financial feasibility and risks
- List of consents and approvals required
- Schedule of additional surveys, tests, analyses, studies and investigations.
- The Guideline Scope of Services and Tariff of Fees for Persons Registered in Terms of the Engineering Profession Act 46 of 2000 (2012) defines the following as within the Normal Scope of Professional Services.
- INCEPTION
- At the inception stage, the client’s requirements and needs are established. The project brief is established and the professional team is appointed. The professional team should contribute towards developing the project brief and concluding the terms of its appointment. Here the professional team should advise on criteria that could significantly impact on the project life cycle cost.
- CONCEPT AND VIABILITY STUDY
- At the Concept and Viability study stage, the preliminary design details and cost estimates should be finalised. This should be concluded in accordance with the project brief.
- A Preliminary design report would include the:
- Concept design
- Process design
- Schedule of design assumptions, required surveys, tests, reports and investigations
- Preliminary design details
- Installation and life cycle cost estimates
- DESIGN DEVELOPMENT / DETAIL DESIGN
- During design development the design team will further develop the concept to realise the following:
- Finalised design
- Detail specification outline
- Financial plan
- Project programme.
- During design development the design team will further develop the concept to realise the following:
- DOCUMENTATION AND PROCUREMENT
- This stage is often combined with the design development stage.
- Its deliverables include:
- Procurement and construction documentation and specifications
- Application of timeous procurement strategies appropriate for the project
- Assisting in the tender evaluation of detailed services and samples for compliance with the design intent.
- CONTRACT ADMINISTRATION AND INSPECTION
- This stage includes the management and administration of the construction contracts and works to facilitate practical completion in accordance with the design intent.
- CLOSEOUT
- Closeout deliverables include:
- Final works-completion lists
- Financial reports and final accounts
- Facilitation in development of Operation and Maintenance Manuals (O&Ms), warranties and guarantees.
- As-built drawings
- Closeout deliverables include:
- INCEPTION
Design Questions
6. In order for the engineer to satisfactorily fulfil the user’s requirements, the following list of questions should be asked, answered and understood by the professional services team.
“Engineers responsible for the design of environmental control systems require guidelines and standards, in order to derive at and to specify appropriate solutions to the problem of building related illness (BRI) in occupied spaces.” -Dr S. A Parsons 2002
- Is the building service required, and why?
- What options are available?
- What is the service’s required performance?
- What is the service’s expected lifespan?
- What is needed in terms of energy management?
- What are the expected service consumption rates?
- What are the expected occupancy profiles per planning unit, considering:
- Patient and staff numbers?
- Peak occupancy times?
- Airborne infection risk profile?
- Seasonal occupancy profiles?
- What are service distribution constraints, considering:
- Location
- Space?
- Fire protection and regulations?
- Services coordination?
- Access for maintenance and operations?
- Repair replacement and refurbishment?
- What are the minimum component/system requirements?
- What are the specific requirements regarding functional controls?
- What are validation and testing requirements
- What are the Maintenance and operational requirements?
- Commissioning and handover requirements
- Special requirements for test and balance documents and certificates
Design considerations
7. Deep buildings
- Deep buildings inevitably result in some measure of ventilation being required within the core areas. Where deep buildings cannot be avoided, the extent of building ventilation can be minimised by planning the deep-core areas as those that require specialist ventilation systems and which could not be served by natural ventilation.
8. Plant and plant room size and location
- Noisy and vibrating equipment shall not be placed near, above or below sensitive areas such as operating rooms and ICUs. They shall be designed and located so as to give sufficient reduction in noise and vibration.
- Plant rooms shall be designed such that there is safe access to equipment for maintenance and repair activities. Plant rooms shall be located away from possible heat and contamination sources.
- Plant rooms shall be located in an accessible area which is secured from unauthorized entry
- Where plant room equipment presents a potential source of airborne contamination (e.g. Legionella and vacuum exhaust) the location of the plant room shall be such that contaminated air is not carried into occupied spaces and air inlets.
Life cycle cost determination
9. When planning and designing building engineering services, the engineer shall take cognisance of the service context within which the facility is placed. As part of the financial plan, outlined in the concept and viability study stage, the engineer will assist in developing the facility’s life cycle cost by giving input into the life cycle cost estimates for the services within the engineer’s responsibility. This financial plan shall be finalised as a deliverable of the detail design stage.
10. Environmental life cycle planning is a critical element of the life cycle planning but should be considered as a service additional to the scope of the normal prescribed services.
Site-survey requirements
11. In order for the engineer to plan adequately, a detailed site survey will need to be conducted to present essential planning information. These factors need to be weighed against the level of service to be provided.
The National Department of Public Works has developed a comprehensive site-survey model for the completion of this task (Citation needed). The following list summarises the information that needs to be developed.
- Geotechnical considerations
- Availability, quantity and quality of mobile phone reception
- Availability, quantity and quality of services such as:
- Electricity
- Water supply
- Drainage conditions
- Gas
- Land and air transport
- Outsourced laundry and catering services
- Proximity to additional social services
Maintenance Considerations
12. Maintenance failures within the building services of the healthcare environment have the potential for severe consequences. Services should be designed with this in mind.
13. The design should consider the financial and environmental impact of disposable and reusable components within the planned maintenance regime. Reporting on the financial aspects of the life cycle plan is required within the normal scope of services of the planning and design project stages.
14. In the development of healthcare building engineering services the designer should consider the following maintenance challenges when designing systems and planning maintenance regimes:
- Where highly specialised services are installed in remote areas, it becomes difficult to source the requisite level of technical skills and, as a result, either maintenance costs rise or the serviceable life of these systems is decreased.
- The availability of spares and contracted technical services becomes problematic in remote locations and this leads to difficulties with unscheduled maintenance and extended callout response times.
- Routine and unscheduled maintenance may need to be performed with a system in operation, with minimal down-time. This should be considered when planning levels of redundancy.
- Routine and unscheduled maintenance should not have a negative impact of the service levels of healthcare. Where IPC and cross-infection risks are high, systems should be designed such that the maintenance staff can complete their work without affecting staff or patient safety.
15. For further guidance on health-facility maintenance, the IUSS Health Facilities Maintenance guidance document should be referred to.
Planning for Retrofitting & Decommissioning
16. While engineering systems may have a functional life of 20 to 25 years, healthcare buildings could have a life of 50 years. It is therefore likely that engineering services would need to be decommissioned, retrofitted, and replaced at least once during the life of a building, and these interventions should be planned for.
17. Projects with a retrofitting element shall include for the formal decommissioning of equipment or services which become redundant or obsolete as a result of the retrofitting project or can be conveniently decommissioned within the project. Decommissioning of any assets shall be undertaken in accordance with the Public Finance Management Act 1 of 1999, the Generally Accepted Accounting Practice, the Companies Act of 2006 and principles of good corporate governance.
18. When planning for retrofitting and decommissioning, consideration should be given to the following aspects:
- Development and implementation of a risk assessment and hazard control plan.
- Identification of clinician and IPC manager with authority to approve or halt construction activities under defined conditions.
- Power requirements for future expansions and installations.
- Emerging healthcare technologies.
- Space for removal and refitting of equipment.
- Materials of construction for recycling potential and disposal.
- Toxicity and environmental impact of gases, paints and polymers.
- Specific healthcare services risks (IPC, etc).
- Occupational Health and Safety Regulations and requirements.
19. A risk assessment shall consider the following aspects:
- Identification of occupancy groups which are susceptible to risks.
- Identification of building services, such as ventilation, in the proximity of the construction activity and the potential impact on function. Specific consideration should be given to specialist ventilation systems.
- Need for supplementary protection or support systems for building services.
- Impact on fire-protection and -response systems, and action plans.
- Impact of noise and vibration on occupants and equipment.
20. Opportunistic environmental or airborne microorganisms and allergens, which are liberated or distributed during retrofitting and decommissioning activities, can present a significant hazard to patients and employees unusually at risk. Where the environmental and risk assessments identify the need for intervention or mitigating controls, the following shall be considered:
- Establishment of rigid non-permeable barriers between patients or staff and construction activities during construction, with the inclusion of appropriate “airlocks” where traffic between occupied and construction areas is required.
- Increased ventilation rates and ventilation efficiency to areas at risk.
- Extraction and filtration systems serving the construction area. Where there is a chance of re-entrainment of diluted exhausted air, a minimum of an EN779-F9 filter should be installed as the final filtration stage. Where air is actively re-circulated it should be filtered with at least an EN1822-H13 final filter.
- Establishment of a protective pressure cascade or airflow direction between zones.
21. For further guidance on the decommissioning of health facilities, the Health Decommissioning and Disposal of Health Facilities and Health Technology guidance document should be referred to.
Sustainability & Environmental Measures
Design Life cycle
Sustainability in designs for new health facilities can be addressed through the following steps:
22. Target setting: Challenging but realistic sustainability targets should be set for the building and agreed with all of the key stakeholders of the project, including the design team, the facilities manager and the funder or owner of the building. Targets should take into account government policy and strategies, as well as local and international best practice.
23. Design principles: Strategies and design principles required to achieve these sustainability objectives should be understood and established from the outset. For instance, energy targets may require passive environmental control strategies to be well understood and established from the outset. These strategies and their implications can be understood through an analysis of best-practice examples and precedents.
24. Integrated design: Once targets and design principles have been established, an integrated design process should be used to ensure that all aspects of the building work together to achieve the required performance. This requires different disciplines to work closely together.
25. Testing: Throughout the design process, checks should be carried out to ensure that the targets set will be achieved. This can be done through calculations, modelling and analysis which assesses performance against targets set. Where aspects of the design are found not to meet targets, a re-evaluation of the design should be carried out and, in an iterative and integrated way, improved to ensure that the performance achieves, or surpasses, targets set.
26. Detailed design and implementation: It is important to ensure that the design principles set out are carried out in detail, or this may affect operational performance. This includes, for instance, seemingly insignificant details such as appropriate locations for switches, labels and instructions.
27. Handover: On completion, effective processes should be followed to ensure that design intentions are carried through into building operation. This includes effective commissioning, handover and training processes which ensure that designers, subcontractors and suppliers transfer knowledge and skills to facilities managers to ensure effective management of the building.
28. Refer to Sustainability Guide for further information on sustainability.
PART - DESIGN SPECIFICATIONS
Design considerations
Best engineering practices for the design, specification, testing and management of wet services, vacuum, medical gases, building electrical, electronic, and lighting and ventilation systems are contained in this guide. This guide also defines applicable local and international informative standards and describes regulatory aspects for consideration.
Heating Ventilation and Air-conditioning
Airborne-Precaution Risk Classification for Healthcare Zones
South Africa does not have a uniform formal policy regarding the classification and design of infection prevention and control zones. Provision of multi-bed patient accommodation and internal waiting areas for out-patients is common practice in South Africa.
| Patient/Staff Susceptibility to Infection** | ||||
|---|---|---|---|---|
| Low | Moderate | High | ||
| Potential for cross infection* | High |
|
|
|
| Moderate |
|
|
| |
| Low |
|
|
controls
| |
For this reason, a burden is placed on the building services design to ensure that the utilities and services provided do not hinder efforts to manage airborne-infection control
The matrix presented above is proposed for consideration when planning mechanical building ventilation for airborne IPC.
Table 24.5 gives further guidance on ventilation rates for specific areas.
For further information regarding the requirements for airborne-infection precaution rooms, refer to Part C, Section 23.3 of this document and the Infection Prevention and Control.
An alternative exposure-based model can be considered where infection risk can be quantified in terms of the environmental reproductive number.
Ventilation requirements
Natural ventilation
Due to the high capital outlay required, medical facilities in countries defined as developing, such as South Africa, are generally not provided with “traditional” engineering control measures, such as ventilation, to achieve acceptable environmental management. -Dr S A Parsons, 2002
- Natural ventilation is driven by a combination of thermo convective or buoyancy effects and wind pressure. Since the drivers of Natural ventilation are inherently variable, natural ventilation has a high variability in effectiveness.
2. In addition to the variability of the drivers of natural ventilation, the responses of the occupants of a space could have a negative impact on the variability of the ventilation system’s performance by opening and closing windows and doors. For this reason it is recommended that, where natural ventilation is considered as the primary ventilation mode, dedicated and controllable ventilation openings be designed and created in the building
3. For additional design guidance on natural ventilation design, the CIBSE Applications Manual AM10 or similar can be consulted.
4. Peak and minimum internal temperatures should be calculated or modelled thermally for a space, for summer and wintertime respectively.
5. The design parameters for internal spaces should be found in the detailed room requirement sheets published in the individual IUSS guidance documents of the various functional units. Where these room requirement sheets are absent or lacking adequate information, the data contained in this document may be used.
6. The following design interventions should be considered for implementation, singly or in combination, in the following hierarchy where the internal design condition cannot be met:
- Reducing solar and internal heat gains
- Using thermal mass to move room temperature extremes to outside of occupancy periods.
- Change occupancy schedules seasonally to improve indoor comfort conditions. (eg. Shift consultation hours from or towards the warmest daytime hours during summer or winter respectively)
- Introducing passive cooling or heating strategies
- Increasing ventilation rates
- Providing mechanical cooling or heating
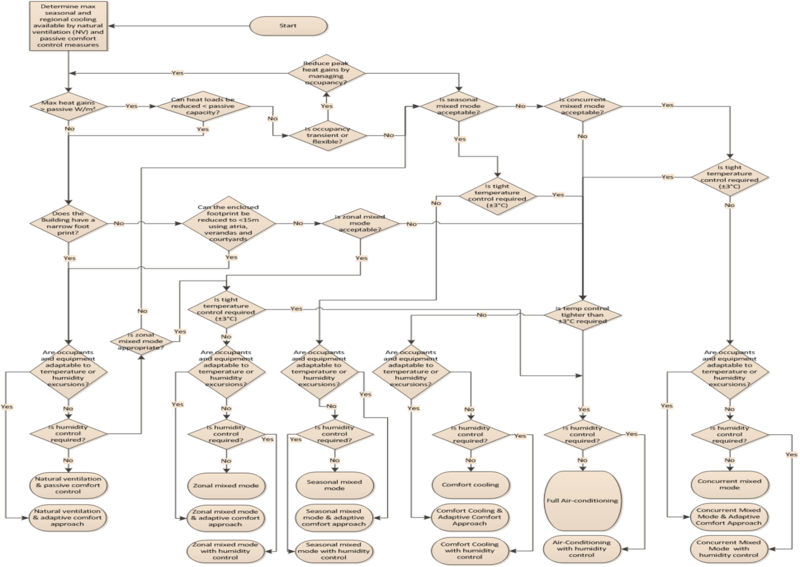
7. Where natural ventilation alone cannot achieve the required air quality, quantity and consistency, mixed mode ventilation shall be considered as a solution preferred over full mechanical ventilation.
8. Mixed mode ventilation is considered as an assisted type of natural ventilation. Here fans are used in combination with damper controlled ventilation openings to ensure minimum ventilation rates are achieved.
9. Where mixed mode ventilation cannot achieve the required air quality, quantity or consistency, mechanical ventilation may be considered as a solution.
Mechanical ventilation and air-conditioning
10. Where the quantity and quality of air within a space can be maintained to a satisfactory degree of consistency, natural ventilation should always be the preferred solution.
11. The design parameters for internal spaces should be found in the detailed room-requirement sheets published in the individual IUSS guidance documents of the various functional units.Where these room-requirement sheets are absent or lacking adequate information, the data contained in this document may be used.
12. Temperature, Relative Humidity (RH) and fresh air requirements
- The adaptive approach to thermal comfort will result in designs with broader acceptable temperature ranges and thereby greater energy efficiency[1]. The following aspects have been found to influence the perception of thermal comfort in a space
- Climate and social custom
- Rate of temperature drift >1°C daily and 3°C weekly
- Exponentially time-weighted mean outdoor temperatures
- For the majority of occupied spaces, unless otherwise indicated, a temperature range of 18-28°C is acceptable, although the level of gowning of the patients and staff needs to be considered in the design
- Clinical practices seldom use explosive anaesthetic gases and the requirement for humidity control from this perspective is generally outdated. Direct humidity control is only required in a select few specialised areas. In general, humidity control is indirect, but the designer should consider the resultant humidity levels and the impact on comfort levels in the space.
Table 3: Specialist ventilation systems, provides a list of spaces that have particular temperature and humidity requirements that are critical to the effective provision of healthcare.
This page or section needs to be cleaned up. |
13. Zoning of a building
13.1. Where the choice between a central and a local ventilation plant needs to be made, the following points should be considered:
- Fire compartmentalization
- Air-handling unit (AHU) sizing
- Duct sizing
- Occupancy schedules
- Occupancy activity levels
- Building, environmental and equipment heat loads
- Airborne contamination control
- Tenancy, functional unit or utility metering
13.2. Zoning of ventilation systems has a large impact on ventilation efficiency and effectiveness.
14. Minimum fresh air requirements
- For minimum fresh air requirements refer to the National Building Regulations and relevant IUSS Infrastructure Guidance Document. Where any apparent conflict between the functional requirements and the “deemed to satisfy” guidance emerges, the rational design route to regulatory compliance would need to be followed so as not to compromise any system’s functionality.
- Where odour control is a consideration, a ventilation rate of 10 litres per second per person may be used.
- Where airborne cross infection is controlled primarily through dilution and natural ventilation, medium and high risk areas require 60 or 160 litres per second per person respectively.
- Where airborne cross infection is controlled primarily through dilution and forced ventilation, medium and high risk areas require 60 or 80 litres per second per person respectively.
15. Ventilation rates
- Air change rates per hour (AC/h) are specified in this document for a room with ceiling height of 3m. Where ceiling heights are increased these rates can be reduced, and vice versa.
- Minimum ventilation rates quoted as air changes per hour should be complied with together with the recommended rate of fresh air per occupant
16. Supply-only vs balanced ventilation systems
- Supply-only ventilation systems do not supply air to all spaces individually, but instead supply air to only the least contaminated or most critical space. Air is then allowed to cascade from the “clean” core to adjacent and auxiliary spaces. Where this type of system is employed, it is critical to be aware of and control the risk of contamination generated in the clean core and permeating through the entire system. This type of system is not appropriate for thoracic and sepsis theatres or areas where unpleasant or noxious odours, fumes and vapours may be generated. It is also important to ensure and prove that the statutory conditions for ventilation and fresh air rates are met for all spaces.
17. Airborne contamination-control concepts
- Airborne contamination control often requires the application of one or more of the concepts described below since airborne contaminants can be generated both internally and external to the controlled zone.
17.2. Barrier concept
- The barrier concept relies on airtight enclosures to isolate the contamination source. Typical examples are glove boxes or barrier isolators.
17.3. Aerodynamic effects
- The displacement concept relies on flushing contaminants away with high volumes of air at relatively low velocity.
- The dilution concept involves reducing contamination levels in a space by diluting them with quantities of "clean" air. The ventilation rate required is a function of the required contamination level, the rate of generation of contaminants in the space, and the ventilation efficiency.
- The pressure-differential concept relies on the pressure differential developed between spaces when "clean" air cascades through small orifices, such as door gaps and pressure-control dampers. The pressure differential, and resulting airflow developed, prevents contaminants from moving into higher pressure “clean” areas from lower pressure "dirty" areas. The following diagram gives indicative values for infiltration and exfiltration rates associated with varying pressure differentials (Pa) and opening sizes (m²).
17.4. Where ventilation rates and fresh air proportions are described within these guidance documents they are to serve as guidance values only. It is the responsibility of the designer to ensure that the ventilation rate selected is appropriate for the specific zone’s operational conditions, occupancy, contamination rates, pressurization, leakage rates, ventilation efficiency and external ambient conditions to achieve the desired airborne contamination and bio-burden levels. Ventilation rates higher or lower than the guidance values may achieve the desired conditions.
18. AIR HANDLING UNITS AND FANS
18.1. GENERAL
- AHUs and fans shall be protected from adverse weather and wind sources which may upset their performance or reliability.
- A detailed name plate shall be included on the air handling units with manufacturer, design air volume, fan speed, cooling capacity, filter data and heating capacity.
- The air handling unit shall include individual differential pressure gauges over each installed filter bank. The gauges shall measure the pressure differential between upstream and downstream of the filter banks. The filter designation and design filter change pressure shall be neatly and clearly marked on each gauge.
- Air-handling units functioning in an airborne contamination control system, and demanding prescribed airflow rates, shall be provided with an electronic control system including variable frequency controllers with direct fan drives to automatically maintain design airflow for all filter conditions. The fans and drives will be selected to maintain design flow rates with all filters at maximum rated pressures.
- Where a ventilation system performs a critical role, air handling units shall have separate electrical distribution boards (Essential and Non-essential). The fan will be on essential power and heater elements may be on non-essential power.
- All compartment doors on the air handling unit shall be clearly labelled
- Where systems require duplicate standby fans or air-handling units these shall be installed with backdraught dampers, sufficiently air-tight for the application (eg EN1751 Cat 3 or 4). Special design and control consideration shall be given to limiting the build-up and dwelling of contaminants in the standby unit.
- AHU’s should be placed in easily accessible plant rooms with sufficient space for maintenance. Access stairs to the plant rooms should permit a technician to easily carry replacement parts or a toolbox into the plant.AHU’s located in ceiling voids are not appropriate or conducive to good operational management.
- AHUs should be designed for a working life of 20 - 25 years
- AHUs shall be designed and positioned such that the largest components, including heating and cooling coils, can be removed and replaced.
- AHUs greater than 1m wide should have hinged access doors large enough to provide full entry. Doors shall be unlockable and openable from the inside of the unit.
- Fan and filter plenums shall be provided with internal illumination and viewing portals such that internal components can be visually inspected without stopping or opening the unit.
- AHUs shall be designed such that dry steam humidification devices can be retrofitted into the systems with minimal disruption and without compromising its performance.
- Ventilation components shall have a drainage or condensate pumping system if they can produce moisture. Drip trays shall be of a corrosion resistant material and drainage systems shall have a 1:20 fall away from the unit in all directions. The unit shall have its own drain trap which shall be sized such that it can function at the fan's full static pressure. The first 3 meters of a dedicated condensate drainage line shall be insulated to prevent condensation within the plantroom. Where a condensate drain services a negative pressure plenum, clear air gaps of 15mm or anti-backflow devices are recommended at the trap discharge into the drainage system.
- Drop stop eliminators in stainless steel frames shall be employed, if fin spacing is less than 10 fins per inch, after the cooling coils in areas with high humidity levels. This includes all coastal areas for off coil temperatures of 10° and less
- In full fresh air systems, primary filters may be situated at the fresh air intake opening of the air-handling unit only if the climate does not require anti-fog or de-icing coils.
- Cooling or heating coils will be protected by a pleated primary filter, rated in accordance with SANS 1424, as minimum.
- In recirculation air systems, primary filters will be situated after the fresh/return air mixing plenum. This arrangement will ensure that blinding filters don’t inhibit the prescribed fresh air proportion.
- The final stage of filters on units serving operating theatres must be located after the supply fan chamber to filter any debris that might come from the fan chamber. HEPA filters should be protected from this potential dust and debris.
18.2. AIR EXTRACTION SYSTEMS
- The design of exhaust systems shall take special consideration of the potential for re-entrainment of contaminated exhaust air into air intakes through, inter alia, openable windows. Where this potential exists, precautionary measures such as aerosol or chemical filtration of exhaust should be applied, as appropriate.
- Single ablution facilities serving private wards or staff do not require extract ventilation, provided there are windows openable to outside.
- Extract ventilation shall be provided by either ceiling extract grilles connected to an in-line ducted fan with outside discharge air grilles by means of galvanized mild steel or PVC tubular ducting or odour extraction system.
- Extract grilles may be of the PVC type with adjustable disc valves, or powder coated or anodized aluminium type with adjustable dampers.
- All multiple toilets shall be provided with ventilation systems, which will serve as an extract ventilation system.
- Single toilet facilities serving private wards for staff do not require extract ventilation, provided there are windows openable to outside
- Where it is not considered safe to enter a space without respiratory protection, the air exhausted from that space should be rendered safe through filtration, decontamination or dilution before discharge.
- The design of exhaust system from airborne precaution areas should be such that all components can be safely maintained during normal service and safely disposed of at decommissioning.
- Planning for disposal of contaminated filters should be as for all biohazardous material.
- Filtration and decontamination components shall be installed upstream of fans, monitoring and control devices.
18.3. ENERGY RECOVERY SYSTEMS
1.3.1. Where full or partial exhaust is required for ventilation systems, energy recovery technologies should be considered. Enthalpy wheels offer a high level of efficiency but introduce a risk of cross infection as the wheel is exposed to both the exhaust and supply airstreams. An enthalpy or energy recovery wheel may only be used if pressure and filtration measures are taken to ensure it is not a potential source of cross infection or re-infection. Energy recovery wheels incorporating purge sections have a markedly reduced efficiency and are not considered to provide sufficient protection against biological cross contamination. Where cross infection is a considered risk, the following conditions shall be met.
- The exhaust airstream shall be consistently maintained at a lower static pressure than that of the supply airstream.
- The exhaust air shall be filtered with aerosol filters upstream of the energy recovery device.
- Levels of filtration, redundancy and safety shall meet the requirements of the biological pathogenicity class in consideration.
19. FILTRATION
- With the exception of the few specialist areas with aero-biological requirements, the primary purpose of filtration is to protect ventilated spaces and ventilation equipment from dust build-up.
- When designing filtration systems serving spaces with a high airborne cross contamination risk, consideration should be given to the safety of maintenance staff that may be required to handle contaminated filters. Where any safety risk is present, contaminated filters should be installed in safe-change or decontaminatable housings.
- All ventilation filter banks should be installed with a means of visually checking the filter pressure across them in Pascals (Pa)
- Filters are classified as being General Fine or Aerosol filters and are to be specified in accordance with the SANS 1424, EN779 or EN1822.
- General filters are selected to remove particles large enough to block cooling and heater fins and settle out of the airstream into the air distribution system. General filters are graded in terms of their “synthetic dust weight arrestance”. General filter grading ranges from G1 to G4. G3 and G4 filters are appropriate for primary air intake and tempered air supply respectively. General filters are not appropriate for combating airborne cross-contamination control.
- Fine filters are selected to keep a ventilated space visibly clean and for the protection of HEPA filters. Fine filters are graded in terms of their “Dust Spot Efficiency” from M5 to F9. F9 filters are capable of arresting particles with the approximate size of some bacteria and can be used for low level cleanrooms (ISO 14644-1 Class 8).
- Aerosol filters are selected for their efficiency in arresting sub-micron particles. They are graded in accordance with their “Most Penetrating Particle Size” (MPPS). Aerosol filters are subdivided into three categories: Efficient Particulate Air (EPA) and High Efficiency Particulate Air (HEPA) and Ultra high Particulate Air (ULPA) Filters in accordance with the EN1822:2009. The table below describes the classification of aerosol filters by integral and local values as defined in the EN1822. SULPA filters are not discussed within this document.
| Group | Filter Class
EN1822 |
Integral Value | Local Value |
| Efficiency % | Efficiency % | ||
| EPA | E10 | 85% | - |
| E11 | 95% | - | |
| E12 | 99.5% | - | |
| HEPA | H13 | 99.95% | 99.75% |
| H14 | 99.995% | 99.975% | |
| ULPA | U15 | 99.9995% | 99.9975% |
| U16 | 99.99995% | 99.99975% | |
| U17 | 99.999995% | 99.999975% |
8. HEPA filter installations shall include both an upstream challenge aerosol injection port and a downstream scan port to facilitate filter challenge testing. ULPA filter installations shall be designed such that an agreed upon test method can be accomplished.
9. All filters used for airborne precaution rooms, theatres or other areas with a high airborne contamination risk shall be selected with a construction suitable for incineration. These filters shall not contain PVC.
10. The installation and testing of HEPA filters shall only be conducted by suitably qualified technicians.
11. HEPA filters shall be specified to be compliant with the requirements of EN1822. Each HEPA filter is to be supplied with an individual factory test certificate displaying that filter's serial number, MPPS rating and DOP arrestance rating.
12. Type-test certificates are only acceptable for EPA and not HEPA filters.
20. HEAT REJECTION EQUIPMENT.
20.1. The location of heat rejection equipment shall be planned such that it does not adversely affect the performance, maintenance or reliability of any related or unrelated equipment, or pose an avoidable health risk.
20.2. The use of evaporative cooling towers shall only be considered where:
- Space, system capacity or efficiency demands their use.
- An effective plan for the control of legionella must be developed and implemented.
21. AIR DISTRIBUTION SYSTEMS
- Discharge from extraction systems shall be located such that contaminated air does not get drawn into any system's air intake of get re-entrained though openable windows.
- The use of internally insulated ducting is not appropriate as internal linings will perish and slough off with aging. Particles from duct linings contaminate final filters and ducting components.
- Flexible ductwork is unsuitable for air distribution in healthcare applications. It should only be used for the final connection to an air terminal and then kept to less than 1.0m. Bends in flexible ductwork should be avoided.
- The use of dampers to throttle a deliberate oversupply of airflow should be avoided. Balance by design is preferable although this will not necessarily reduce the total fan pressure. Use of adjustable blade dampers should be kept to a minimum as these items may drift, can be tampered with and increase the complexity of commissioning. The use of constant volume dampers may improve stability of volume critical systems but may also mask inefficient design and be the source of increased system noise.
- Cleaning and access doors are to be installed in all air distribution ductwork to facilitate:
- Cleaning
- Inspection
- Measurement
- Maintenance
21.6. Ductwork installations shall be designed, built, installed and commissioned in accordance with SANS 1238: Air-conditioning ductwork and SANS 10173: The installation, testing and balancing of air-conditioning ductwork
22. ELECTRONIC CONTROLS
- Location of sensors in ventilation systems should ensure that the temperature and humidity measurement for monitoring control is representative of the occupied area
- The humidifier control will include humidity monitoring of the mixed airstream downstream of the humidifier lance and shall prevent this airstream from approaching dew point.
- The use of variable speed drives (VSD) can save energy in systems operating under varying motor loads. Reducing the fan speed when filters are new or clean can result in considerable energy savings over the life of a system. Reliability of smaller sized VSDs is a potential drawback, and for this reason VSDs must be selected and sized for high service life. VSDs should also be installed such they can be bypassed and the system can be run in manual control while failed VSDs are replaced or repaired. When designing with variable speed drives, cognisance should be taken of a motor’s minimum cooling requirements and the maximum restart rate. Caution should be exercised where variable speed drives are used in conjunction with constant volume dampers or volume flow controllers. This combination could drive up total system pressure where duct total pressure as opposed to velocity pressure is used as control feedback. Additional requirements for the selection of drives for variable air volume (VAV) fans is described in SAN204:2011
- Plant control systems should incorporate start-up and shut-down sequencing logic to prevent flow reversals and overheating.
- Set-back controls should be considered for spaces that have intermittent occupancy. This feature should be used with caution in specialist areas as poorly considered set-back conditions could compromise containment or contamination control.
- Where more than one ventilation system serves a department, system interlocks may be required to prevent unwanted airflow reversals during system shutdown or failure.
- Operational status indicators should be displayed locally in areas served by ventilation systems.
- "Low Air Flow" and "Plant Failure" alarms should also be installed in a location which can be manned by relevant and trained staff.
- Electronic control systems should be developed using recognised open protocols and standards such as BACnet DeviceNet, LonWorks, Modbus, SOAP and XML.
23. SPECIALIST VENTILATION SYSTEMS
23.1. The following areas will require specialist ventilation systems:
Table 3 Specialist Ventilation Systems
| Department Name | Ventilation system type |
| Operating departments | Clean and Ultra clean ventilation systems: ISO8 to ISO5 & UDAF |
| Obstetrics | Clean ventilation systems ISO8 |
| High care, Critical Care and Intensive Care | Fine filtered ventilation or Clean ventilation systems: Unclassified - ISO8 |
| Isolation units | Negative pressure ventilation, no recirculation |
| Pathology labs | Biosafety ventilation: (BSL2 – BSL4) |
| IVF Labs | Clean ventilation systems: VOC Filtration |
| Burns units | Clean ventilation systems/ Negative pressure ventilation/ RH control |
| Neonatal Units | Dedicated ventilation systems/ RH control |
| Mortuary unit | Cold Rooms/ Extraction systems/ Odour Control |
23.2. Broad requirements for these systems are in this document. The engineering team shall consult each department’s specific design guidance document for detailed requirements.
23.3. AIRBORNE PRECAUTION ROOMS (INCLUDING TB)
- Where specific diseases are considered in the design of an airborne precaution room, the US CDC's "Select Agent List" may be consulted for design guidance until a South African list is compiled.
- Airborne precaution rooms shall be ventilated with a minimum of 12AC/h of fresh air or uncontaminated air. High risk areas such as sputum booths and airborne diseases wards shall have a nominal ventilation rate of 80 ℓ/s per person..
- Medium Risk areas such as congregate spaces such as waiting areas shall have a nominal ventilation rate of 60ℓ/s per person.
- Mechanical ventilation may be employed to achieve the minimum ventilation rates. It should be noted that very high ventilation rates can be achieved by employing a well-considered natural ventilation design. Consideration may also be given to mixed mode ventilation systems, which combine mechanical and passive ventilation and temperature control. An open window policy may therefore be adopted, with careful consideration of all the associated cross infection risks and management challenges.
- Airborne precaution rooms shall be designed so as to provide thermal comfort. Where occupants have freedom in location and dress code, an adaptive thermal comfort model should be adopted. Heating, cooling and energy recovery devices shall pose no risk of harbouring pathogens or increasing the cross-infection risk.
- Air from the airborne precaution rooms shall not flow into adjacent, uncontaminated rooms or adjacent airborne precaution rooms. Air shall not flow from a room with a higher airborne infection risk category to a room with a lower risk category.
- Ventilation ducting and pipe work shall not form a conduit by which pathogens can transfer from one zone to another whether the ventilation system is running or not. Filtration devices and anti-backflow devices may be employed provided these do not pose a risk of infection to maintenance staff.
- Filtration requirements for supply and exhaust air should follow the bio-containment requirements of that select agent being contained.
- The location of supply and air terminals should be such that the airflow patterns generated within the room serve to suppress and remove airborne particles.
- For general waiting areas or where the pathogens are known and unlikely to pose an environmental risk, exhaust air filtration may not be required provided exhausted air is directed 3m away from open-able windows and air intakes and there is no risk of re-entrainment of this air. See section 18.2.
- Commissioning and validation shall be well planned, diligently executed, fully documented and approved by suitably experienced professionals. It is advisable to have the validation process conducted or approved by a party independent of the designer and installer.
- Numerical or physical modelling may be of value in the design and validation process.
23.4. OPERATING THEATRE VENTILATION DESIGN
23.4.1. GENERAL REQUIREMENTS
- Constant volume systems shall be employed to maintain the correct pressure with respect to any adjoining rooms. The contamination control concept shall be developed in accordance with ISO14644-4
- Temperature range shall generally be 18°C to 24°C with a minimum relative humidity of 45% unless otherwise specified
- A pressure differential of 10-15Pa is to be maintained between the theatre and adjacent rooms when all doors are closed.
- Theatres may be maintained at a room pressure positive or negative to the adjacent rooms depending on the contamination control requirements.
- A theatre’s room pressure shall always be positive relative to technical spaces.
- Negative pressure theatres should not employ recirculation of room air.
- Minimum fresh air requirements are 5-7 Air Changes per hour to satisfy the occupancy requirements.
- Additional fresh air may be required for pressurisation and shall be designed to maintain the required pressure differential between the theatre, the ancillary rooms and the corridors. The fresh air rate shall be selected to offer the required pressurisation at the greatest possible energy efficiency. Rules of thumb rates of percentage fresh air as a function of the supply air rate are not acceptable methods for determining additional fresh air requirements for pressurization.
- Separate temperature controls in each theatre are to be provided.
- No manual on and off switching of air handling plant to be done from within the theatres.
- Automatic motion sensors / thermal sensors to ensure that the units are switched on when there is a presence in the theatre. Theatre ventilation switching may be linked to the theatre unit’s lights.
- Automatic switching of ventilation system to incorporate run-on timers to prevent overheating and accidental shutdown.
- An additional override to be used to switch the units on when the temperature in the theatre exceeds 25°C for the protection of stored medicine
- For ISO7 and cleaner areas, HEPA filters shall be mounted within the supply air terminals and UDAF plenums.
- These validation tests shall be performed in accordance with SANS 14644Parts 1, 2 and 3 at the recommended intervals (SANS 14644-2) or after any system or building intervention has been completed. Detail records are to be kept and be presented upon demand.
- No internal ducting insulation is permitted.
- In multi theatre suites it is advisable to have dedicated AHUs per theatre. [TR1]
23.4.2. UNIDIRECTIONAL AIRFLOW OR ULTRACLEAN THEATRES
- Airborne particulate contamination levels are not to exceed ISO 14644-1 Class 5 under protected zones (UDAF and Setup area) and ISO 14644-1 Class 6 in background and ancillary areas. These conditions are to be achieved under operational conditions.
- Temperature range shall generally be 18°C to 24°C and relative humidity 45% to 60% unless otherwise specified
- Ultra-clean theatre ventilation shall not be completely shut down when unoccupied unless required for maintenance interventions. Ventilation systems serving UDAF plenums shall instead switch to a minimum velocity set-back mode to prevent contaminants settling underneath the UDAF screens.
- Delivery of the conditioned air shall be by downward movement from the ceiling to four low level exhaust outlets located near the corners.
- All ductwork between HEPA filter housings and air terminals shall be high pressure rated and constructed of galvanised sheet metal. In the final connection to the terminal, where alignment necessitates, a maximum of 300mm of thermally insulated, high pressure flexible ducting may be used.
- The Air Conditioning system is to be complete with G4 primary, F9 secondary and H13 HEPA Filters.
- The ventilation systems shall be designed with a mean air velocity of between 0.35 & 0.45 m/sec measured below the UDAF screen and at the working height.
- Refer to ISO14644-4 for guideline ventilation rates for balance of areas.
- The mean velocities below the UDAF screen and at the working height shall not differ by more than ±10%
- A standard size of the UDAF screen is 2400 x 2400mm. The required size could vary dependent on the layout and function of the operating theatre.
- The protected zone below the UDAF plenum shall be clearly demarcated on the floor
23.4.3 CLEAN OR MAJOR THEATRES
- Airborne particulate contamination levels are not to exceed ISO 14644-1 Class 6 in all protected areas and Class 7 in background areas. These conditions are to be demonstrated as achievable under operational conditions.
- The ventilation system is to include G4 Primary, F9 Secondary and final H13 HEPA filters. No air tempering and conditioning equipment is permitted downstream of the HEPA filters
- The conditioned air is to be introduced into the theatre via suitable diffusers.
- Refer to ISO14644-4 for guideline ventilation rates.
- Additional fresh air may be required for pressurization and shall be designed to maintain the required pressure differential between the theatre, the ancillary rooms and the corridors. The fresh air rate shall be selected to offer the required pressurization at the greatest possible energy efficiency.
- These validation tests shall be performed in accordance with SANS 14644 Parts 1, 2 and 3 at the recommended intervals (ISO14644-2) or after any system or building intervention has been completed. Detail records are to be kept and be presented upon demand.
- All ductwork between the HEPA filter housing and the air terminal shall be of rigid medium pressure ducting (SANS 10173) construction. All ductwork upstream of the HEPA filter housing shall be rigid high-pressure ducting. Where alignment necessitates, the final connection to the terminal shall have a maximum of 300mm of thermally insulated flexible ducting.
- Recirculation from the theatre room to zones outside of the theatre room is not permitted and all recirculated air shall be filtered through the secondard and HEPA filters as a minimum. Recirculation from zones outside of any theatre room into that theatre room is not permitted.
23.4.4. MINOR THEATRES
- Airborne particulate contamination levels are not to exceed ISO 14644-1 Class 8 in all areas. These conditions are to be achieved under operational conditions.
- For recirculation systems the ventilation system is to include G4 Primary, F9 Secondary and H13 HEPA filters.
- For single pass systems the ventilation system is to include G4 Primary and F9 Secondary filters.
- The conditioned air is to be introduced into the theatre via suitably sized diffusers.
- Refer to ISO14644-4 for guideline ventilation rates.
- All ductwork between the HEPA filter housing and the air terminal shall be of rigid medium pressure ducting (SANS 10173) construction. All ductwork upstream of the HEPA filter housing shall be rigid high pressure ducting. Where alignment necessitates, the final connection to the terminal shall have a maximum of 300mm of thermally insulated flexible ducting.
- For ISO7 and cleaner areas, HEPA filters shall be mounted within the supply air terminals and UDAF plenums.
VALIDATION OF SPECIALIST VENTILATION SYSTEMS
- Validation testing shall be completed in accordance with national standards for standardized tests (eg ISO14644 and ISO 14698 for cleanrooms) and shall be completed against mutually agreed protocols for non-standard tests.
- It essential that the validation testing of a ventilation system’s contamination control performance parameters is conducted against operational, and not only "at-rest", conditions. Validation against "as-built" conditions offers little insight into the ultimate performance of the system.
- Pre-Commissioning Checks shall cover the following aspects prior to the commencement of formal commissioning:
- Check whether the Design Specification satisfactorily addresses the demands of the User Requirement Specification.
- Check whether the ventilation systems have been provided and installed in accordance with the design specifications and drawings
- Check that the buildings either housing or served by the ventilation equipment is complete and finished such that testing can commence safely and effectively.
- Check that all AHUs, chillers, heat rejection equipment and filters are sufficiently accessible for inspection and maintenance.
- All components are connected and are functional
- Door gaps and openings are installed and sized as specified in specialised zones
- Airflow control devices are installed in the correct locations and in the correct orientation
- Duct and filter tests ports are installed and sealed satisfactorily
- Safety and control interlocks are established
- Fan and drive guards are in place
- Safety and warning signs are in place
- All major system components or sub-systems are clearly labelled with functional or controls identification in a neat and durable fashion.
- Fluid and air pressure monitoring gauges are labelled with identification and acceptable maximum and minimum operating conditions.
- All wiring, piping and ducting colour banding is complete in accordance with SANS-1091
- CLEANLINESS CHECKS:
- AHUs shall be checked for cleanliness on internal plenums with special attention being paid to fan and discharge plenums and condensate drip trays and drain lines.
- Ducting serving “clean” areas shall be cleaned prior to installation and the ends shall be sealed until installation. Open ends of duct runs shall similarly remain sealed during construction. Spot checks for compliance during the installation process are recommended.
- Recommended Filtration Levels and Ventilation Rates for Mechanically Ventilated Areas
This table serves as a quick reference guide and will be revised as and when detailed room data sheets are developed within each department’s guidance documents.
| Systems Serving[TvR1] : | Primary
Filters |
Secondary
Filters |
Secondary
Filters |
Tertiary
Filters |
Airflow Type | Airborne Particle Count | Ventilation[1]
(Considering Forced) |
Temperature | ||
| Pleated Panel | Pleated Panel/ Bag | High Capacity
Rigid Minipleat |
High Capacity
Rigid Minipleat |
Unidirectional/
Turbulent/ Mixed |
Protected zone | Background area | Minimum
Outdoor Air |
Min. Air changes
per Hour |
Design Range[2] | |
| EN779 Classification
G4 |
EN779 Classification
F6 |
EN779 Classification
F9 |
EN1822 Classification
H13-H14 |
U/T/M | SANS14644-1
Class |
SANS14644-1
Class |
ℓ/s per person | (Assuming 3m ceiling height) | °C | |
| Casualty/Minor Stitch Procedure room | X | X | T | 7.5 | 20 | |||||
| Theatres: Maternity/Caesarean | X | X | X** | T | NA | 7 | 7.5 | 20 | ||
| Theatres: General Surgery | X | X | X** | T | NA | 7 | 7.5 | 20 | ||
| Theatres: Gynaecology | X | X | X** | T | NA | 7 | 7.5 | 20 | ||
| Theatres: Ophthalmology | X | X | X** | T | NA | 7 | 7.5 | 20 | ||
| Theatres: Urology | X | X | X** | T | NA | 7 | 10 | 20 | ||
| Theatres: Endoscopy | X | X | X** | T | NA | 7 | 80 | 20 | ||
| Theatres: Plastic Surgery | X | X | X | T | 6 | 7 | 7.5 | 70*** | ||
| Theatres: Bone Surgery/Orthopaedic | X | X | X | M | 5 | 7 | 7.5 | 70 | ||
| Theatres: Thoracic | X | X | X | M | 5 | 7 | 80 | 70 | ||
| Theatres: Vascular??? | X | X | X | M | 5 | 7 | 7.5 | 70 | ||
| Theatres: Neuro Surgery | X | X | X | M | 5 | 7 | 7.5 | 70 | ||
| Waiting and Congregate Areas | X | 60 | 8 | 18-28 | ||||||
| Auditoriums | X | 7.5 | 4 | 22-26 | ||||||
| Mortuary | X | X | 12 | 22-25 | ||||||
| Bath Room | 25 | 10 | N/A | |||||||
| Dirty Utility Room | 40 | 10 | N/A | |||||||
| Blood Bank | X | 4 | 22-25 | |||||||
| Casualty | X | X | 7.5 | 12 | 22-25 | |||||
| CSSD | X | X | X | 7.5 | 20 | 22-25 | ||||
| Dark Room | X | 10 | 22-25 | |||||||
| Dining Rooms/Canteens | X | 7.5 | 10 | 18-28 | ||||||
| General Stores | X | 4 | N/A | |||||||
| Laboratories | X | X | 6 | 22-24[3] | ||||||
| Labour/Delivery Room | X | 4 | 22-24 | |||||||
| Laundry – General | X | 7.5 | 10 | N/A | ||||||
| Lecture Halls | X | 7.5 | 4 | 22-26 | ||||||
| Outpatients Departments | X | 60 | 4 | 18-28 | ||||||
| Pharmacy Dispensing | X | X | 7.5 | 4 | 22-24 | |||||
| Pharmacy Store | X | X | 7.5 | 4 | 22-24 | |||||
| Specialist Clinics- ENT | X | X | 7.5 | 4 | 22-26 | |||||
| Sterilizer Equipment | X | 7.5 | 10 | N/A | ||||||
| Toilet Room | 7.5 | 10 | N/A | |||||||
| Units: Treatment Room | X | 7.5 | 6 | 24-26 | ||||||
| Units: Burns | X | X | X | T | 8 | 8 | 7.5 | 20 | 26-28
(50-60%RH) | |
| Units: HCU / CCU | X | X | 7.5 | 30 | 22-24 | |||||
| Units: ICU | X | X | 7.5 | 30 | 22-24 | |||||
| Units: ICU Neonatal | X | X | 7.5 | 6 | 26-28 | |||||
| Wards: General | X | 7.5 | 4 | 18-28 | ||||||
| Wards: Airborne Precaution Rooms/Isolation[4] | X | X | 80 | 12 | 22-24 | |||||
| Wards: Maternity | X | 7.5 | 4 | 24-26 | ||||||
| Wards: Medical | X | 7.5 | 4 | 24-26 | ||||||
| Wards: Paediatric | X | 7.5 | 4 | 22-25 | ||||||
| Wards: Psychiatric | X | 7.5 | 4 | 20-28 | ||||||
| Wards: Orthopaedic | X | 7.5 | 4 | 20-28 | ||||||
| Wards: Surgical | X | X | 7.5 | 4 | 20-28 | |||||
| Wards: TB | X | 80 | 12 | 20-28 | ||||||
| Radiology: General | X | X | 7.5 | 6 | 22-24 | |||||
| Radiology: Airborne Precaution | X | X | 60 | 6 | 22-24 | |||||
| Radiology: MR/CT
Scanner |
60 | 6 | 2-24 | |||||||
[1] These rates are considered for forced ventilation systems only. Average natural ventilation rates may be higher
[2] Temperature range not to be exceeded for more than 50 hours per year.
[3] Specialist cleanrooms and laboratories may require lower temperatures.
[4] Levels of filtration are dependent on pathogenicity. Exhaust filtration may also be required.
Commissioning tests shall include and record, but not be limited to:
| System | Commissioning Test | Special Instructions |
| All | Standard of installation | Test to be authorised by client's representative. |
| Air Handling Units and Fans | Fan motor drive speed and rotation. | Cognisance should be taken of motor cooling requirements |
| Fan motor current draw. | - | |
| HEPA Filter challenge testing. | In accordance with ISO DIS 14644-3 | |
| AHU heating and cooling coil performance | Report on-coil and off-coil air conditions for full heating and full cooling with no air bypass. | |
| AHU leakage tests | - | |
| Heating and Chilled Water circuits have been charged, dosed and pressure tested | - | |
| Flow rates and pressures across heat exchangers, pumps and compressors | Pressure drops and flow rates should be measured, recorded and confirmed to be in accordance with design specifications. | |
| Room conditions | Airflow and room pressure balancing in accordance with design tolerances. | In accordance with ISO DIS 14644-3 Acceptance criteria Normally +10-0% |
| Room Temperature and humidity | - | |
| Air Distribution systems | Dampers and registers shall be locked and marked after balancing | - |
| Duct leakage tests for medium and high pressure ducting in accordance with SANS 10173 or DW/143 requirements, as agreed upon | - | |
| Water Distribution Systems | Pressure Drops and Flow Rates | Pressure drops and flow rates should be measured, recorded and confirmed to be in accordance with design specifications. |
| Control System | Control system loop and function checks | - |
| Alarm Checks | - | |
| System Start/Stop sequencing checks | - | |
| System Set-Back mode checks | Include room condition and contiguous system impacts |
24.7. Select validation tests shall be conducted at intervals defined by the client:
| Tests | Required/ Optional | Recommended Frequency of testing | At-Rest or In-Operation Testing |
| Airflow volume tests | Required | 12 months | At-Rest |
| Airflow visualization
(Airborne precaution rooms) |
Required | 12 Months | At-Rest & In-Operation |
| UDAF Velocity Tests | Required | 12 Months | At-Rest & In-Operation |
| UDAF Airflow Visualisation | Required | 6 Months | At-Rest & In-Operation |
| Room pressure tests | Optional | 3 Months | At-Rest & In-Operation |
| Airflow direction tests | Required | 1 Month | At-Rest & In-Operation |
| Discreet practice counts | Required | 12 Months | In-Operation |
| Bio-burden testing | Required | 1 Month | At-Rest |
| Filter challenge testing | Optional/Recommended | 24 Months | At-Rest |
| Room Condition recovery | Required | 24 Months | In Operation |
24.8. Prescribed validation reports shall include:
- References to the test protocol
- Acceptance criteria
- Test results
- Test equipment identification and calibration status
- Name and signature of tester
- Name and signature of facility representative
- Dates of test and acceptance by client
Medical gas installations
25. The design parameters for internal spaces should be found in the detailed room requirement sheets published in the individual IUSS guidance documents of the various functional units. Where these room requirement sheets are absent or lacking adequate information, the data contained in this document may be used.
26. All units of a health establishment, except sub-acute and hospice facilities, where patients are accommodated and treated, must have medial gases and vacuum provided by medical grade piped services, with indexed terminal connecter points. Bottle systems may be provided in sub-acute and outpatient facilities.
27. Mobile gas services must be available for crisis situations.
28. Sub-acute facilities must have one mobile oxygen cylinder per 10 patients and one suction machine for every 10 patients.
29. The minimum services to be supplied to all Acute Care areas are described in Table 11.1. Should the data in this table be in conflict be the table presented in the individual departmental design guidance documents, those individual guidance documents take precedence.
| Description | Oxygen | HP Air | LP Air | N2O | VAC | Scavenging | |
|---|---|---|---|---|---|---|---|
| Major Theatre8 | Theatre Panel | 1 | 1 | 1 | 1 | 2 | |
| Per Pendant | 2 | 2 | 2 | 1 | 2 | 1 | |
| Minor Theatre8 | Theatre Panel | 1 | 1 | 1 | 1 | 2 | |
| Per Pendant | 2 | 2 | 2 | 1 | 2 | 1 | |
| Cath Lab8 | Theatre Panel | 1 | 1 | 1 | 1 | 2 | |
| Per Pendant | 1 | 1 | 1 | 1 | |||
| Post Op | Bedhead
Trunking |
1 | 1 | 1 | |||
| Procedure
Room |
Theatre Panel | 1 | 1 | 2 | |||
| Resuscitation Bay | 1 | 1 | 2 | ||||
| Delivery Room | 2 | 1 | 2 | ||||
| High Care Unit, Per Bed | 1 | 1 | 2 | ||||
| Intensive Care Unit Per Bed | 2 | 2 | 2 | ||||
| Casualty Per Bed | 1 | 1 | 1 | ||||
| Wards | 1 per
2 beds |
1 per
2 beds |
30. A gas alarm system to monitor gases, excluding scavenging, must be installed in a location that is manned 24 hours per day. A slave panel must also be installed in the intensive care unit and in the theatre complex. This alarm system must be connected to UPS.
31. All piped vacuum and oxygen systems must have mobile back-up systems with adequately trained staff to handle them. . The back-up service shall be automatically activated if the line pressure drops below the set operating pressure. All back-up services and change-over valves shall be on UPS and diesel generator supplies.
32. Medical air (low pressure) for respiratory purposes must be provided at a fixed pipeline pressure of 400 kPa. Medical air (high pressure) for driving surgical power tools must be provided at a terminal usage pressure between 70 0kPa and 1000 kPa, depending on the tools/equipment to be used. ICU and operating rooms must be provided with a back-up system for both low and high pressure service. Air compressors must be fed off standby power supply.
33. Anaesthetic gas scavenging, which is a low-pressure suction system that removes exhaled anaesthetic gases from the patient circuit must be provided. Each outlet point must have its own balancing valve to allow the system to be balanced progressively from the furthest outlet point towards the suction fan or pump.
34. The vacuum installation shall comply with SANS 7396-1. Vacuum liquid bottle traps must be installed to collect any blood/fluid etc. that may be drawn into the pipeline. One bottle trap per operating room, ICU, ward block and other patient unit, must be supplied. Where possible the vacuum trap should be located in a sluice room. Emergency suction facilities must be provided in the ICU and High Care, operating rooms, recovery room, delivery room, emergency unit and nursery, and must be available to all patient rooms. Bacteria filters must be installed in the vacuum main before the vacuum reservoir and pumps. Used filters are considered a bio-hazard and must be handled accordingly when being changed and disposed. Care must be given to the location of the exhaust discharge of vacuum plants taking into account locations of windows and other air inlet points. Vacuum pumps must be fed off standby power supply.
35. Gas service isolation valves should be carefully positioned for each clinical unit to avoid shutdowns of major sections.
36. Gas service outlets to be identified and colour-coded with 3mm lettering.
37. Should compressed air operated autoclaves be employed, High Pressure medical air may be taken to such equipment, provided the system possesses sufficient capacity.
38. Should pendants requiring compressed air for aid of movement be employed, High Pressure Medical Air may be taken to them, provided the system possesses sufficient capacity.
39. Should Health Technology Workshops require medical gas outlets for testing and servicing of medical equipment, the required service may be taken to them, provided the system possesses sufficient capacity.
40. SANS 7396-1, as amended, specifies the requirements from design to commissioning of medical gas and vacuum systems
41. Medical gas and vacuum pipelines shall be marked in accordance with SANS 7396-1 and ISO 5359 as applicable
42. SANS 7396-2, as amended, specifies the requirements from design to commissioning of anaesthetic gas scavenging disposal systems.
43. Colour coding of anaesthetic gas scavenging disposal system shall be red magenta or in accordance with the national standard. An example of red magenta is 3050-R40B, in accordance with SS 01 91 02.(Refer to SANS 73962-2).
44. Colour coding of non-medical gas piping must be as per SANS 10140-3:2003.
45. SANS 1409, as amended, specifies the requirements for non-interchangeable outlet sockets and probes for specific medical (gas and vacuum) services used in hospitals.
46. Plain ended copper tubing for low pressure medical gas and vacuum shall comply with the requirements of SANS 1453 and SANS 1067-1 or SANS 1067-2, as deemed suitable.
47. Laboratory gas taps and valves shall be marked as described in SANS 10140-4
Electrical installations
48. Lighting in Hospitals
48.1. The design parameters for internal spaces should be found in the detailed room requirement sheets published in the individual IUSS guidance documents of the various functional units. Where these room requirement sheets are absent or lacking adequate information, the data contained in this document may be used.
48.2. Within the available scope presented in the National Building Regulations, the following lighting requirements should be interpreted with the aim of maximum energy and cost efficiency. The following innovations could be adopted to achieve this aim:
- Daylight harvesting with passive building elements and active systems response.
- Adoption of task lighting, where appropriate, within the scope of present and future planned activities.
- Considered selection of lighting elements and solutions.
- Considered selection of internal colours and materials.
- Accommodation for visually impaired occupants.
48.3. Where a requirement for natural light (daylight) is stated, this may be met if the room opens onto an atrium or courtyard, or if a roof light is incorporated, provided that privacy within the room or space is maintained. In addition, daylight may be borrowed from an adjacent room by means of glazing the wall in between, provided that the adjacent room or corridor is within the same unit.
48.4. Save where otherwise provided for in the requirements, health establishments must comply with the following: (Adapted from CIBSE Lighting Guide 2: Hospitals and Healthcare settings).
Table 6 Levels of Indoor Lighting (Adapted from CIBSE Lighting Guide 2: Hospitals and Healthcare settings)
| Area, unit or department | Service illuminance
/ lux |
Max. point illuminance/ lux
(not to be exceeded) |
Unified
Glare Rating (UGR) |
Min.Ra | Measurement
Point |
Type of control | Standby lighting level (%) |
| Common areas
- changing room - chapel - classroom - consulting room (general) - care room (deep plan) - day room - disposal (clinical, domestic waste) - doctor’s office - domestic services room - drug store (ITU/HDO) - general office - seminar room - seminar room - staff change - staff rest room - utility room (clean) - utility room (dirty)
|
100-150 300 300 300 200 200 500 100 500 300 100-150 300 100 50/200 150 200
|
n/a 520 520 520 350 350 850 170 850 520 n/a 520 170 n/a 260 350
|
22 19 19 19 22 22 19 19 19 19 19 19 22 22 19 22
|
80 80 80 80 80 80 80 80 80 80 80 80 80 90 80 80
|
Pews Desk Work Surface Work Surface Work Surface Floor Desk Floor Desk Desk Floor Work Surface Floor Work Surface Work Surface Work Surface
|
Normal Normal Normal - - Normal Normal Normal Normal Normal Normal Selective Normal - Normal N
|
- - >30% - - - >30% - >30% >30% - >30% - - >30% >30%
|
| Corridors (screened from bed bays)
- by day - by night
|
200 5-10
|
350 n/a
|
22 22
|
80 80
|
Floor Floor
|
S Normal
|
>30% >30%
|
| Circulation/communal areas
- corridors (general) - day room - entrance canopy - entrance lobby - hairdressing salon - hospital street - library - lift car - lift lobby - loading bay - reception area - relatives overnight - rest area - shop/kiosk - storage (general) - toilets |
200 n/a n/a 300 200 300 150 200(min) 100 300 150 150 300 200 200 |
350 n/a n/a 520 350 520 260 n/a 170 520 260 260 520 350 n/a |
- - 22 - 19 19 - 19 22 19 - 19 - 22 22 |
80 80 80 80 80 80 80 80 80 80 80 80 80 80 80 |
Floor Road surface Floor Chair Floor Desk Floor Floor Platform or floor Floor Work Surface Floor Counter Floor Floor |
S Selective Selective Normal N/EM Normal Sp Selective Normal Normal Normal Normal Normal Normal Normal |
>30% >30% >30% - >30% - - >30% - >30% - - - - - |
| Restaurant/catering/breakout areas
- beverage bay - counter - general - servery - tables - washing up
|
100 300 50 300 50/200 |
170 520 100 520 n/a 520
|
- 22 - 22 - 22
|
80 80 80 80 80 |
Floor Counter Floor Counter Tables Sink
|
Normal Normal S Normal Normal Normal
|
>30% - >30% - - >30%
|
| Wards and bedded areas
- children’s play area - circulation space - circulation space (night) - examination/treatment |
170 10 1000(local) |
170 10 n/a |
19 - - |
80 80 90 |
Floor Floor Bed level (usually provided by examination lamp |
Normal Normal Normal |
>30% >30% >90% |
| Area, unit or department | Service illuminance
/ lux |
Max. point illuminance/ lux
(not to be exceeded) |
Unified
Glare Rating (UGR) |
Min.Ra | Measurement
Point |
Type of control | Standby lighting level (%) |
| Nursing
- general nursing care/examination - night light - nurses’ station (day) - nurses’ station (night) - observation/night watch - observation/night - mental illness care wards - patient reading (adult) - reading lights - ward corridors (day) - ward corridors (night) |
5 300 30/200 20 1 to 5 200 300 300 200 50 |
10 520 250 40 n/a 350 520 520 350 75 |
- 19 22 - - 19 19 19 19 19 |
80 80 80 80 80 80 80 80 80 80 |
Work Surface Desk Work Surface Bed head Bed head Floor Bed head Patient activity area Floor Floor (50% uniformity required)
|
Normal Variable Normal Normal N/Sp Normal Normal Selective Normal Normal |
>90% >30% >90% - >30% >30% >30% >30% >90% >30% |
| Orthopedic
- pacemaker - treatment (general) - venesection
|
300 300
|
520 520
|
19 19
|
80 80
|
Work Surface Chair
|
Normal Normal
|
>30% >30%
|
| Critical care
- intensive care (night) - observation/night watch - high dependency unit (HDU) - intensive care unit (ICU) - bed head (day) - night light - simple observation/examination - examination
|
20 100 100 30 to 50 5 to 10 300 1000 (local)
|
40 170 170 n/a 10 520 n/a
|
- 19 19 22 - 19 -
|
80 80 80 80 80 80 90
|
Bed head Circulation/general Circulation/general Bed head Bed head Bed Bed level(to be provided by examination lamp) |
N/Sp Normal Normal Normal Normal Selective Normal
|
>30% >90% >90% >90% >90% >90% >90%
|
| Coronary care
- bed head (day) - observation/night watch - simple observation/examination - examination
- staff base (day) - staff base (night)
|
5 to 10 300 1000 (local)
300 30/200
|
n/a 520 n/a
520 250
|
- 19 -
19 19
|
80 80 90
80 80
|
Bed head Bed Bed level (to be provided by examination lamp) Desk Desk
|
Normal Selective Normal
Selective Variable
|
>90% >90% >90%
>90% >90%
|
| Nurse’s station/staff base
- day - night - interview
|
30/200 300
|
250 520
|
19 19
|
80 80
|
Desk Desk
|
Variable Normal
|
>90% >30%
|
| Operating theatres
- anesthesia (examination) - anesthesia room (general) - angiography room - endoscopy - operating room general - operating table/cavity - porter’s area - post anaesthesia recovery - preparation - scrub up - transfers - utility rooms
|
1000 (local)
500 500 300 300 1000 10000 to 100000 300 500 500 500 300 100 to 150 |
850 850 520 1500 n/a 520 850 860 860 520 n/a |
19 19 19 19 - 19 19 19 19 19 19 |
80 80 80 90 90 80 90 801 80 80 80 |
Work Surface Work Surface Work Surface Work Surface Work Surface Trolley/bed Work Surface Bench Sink top Work Surface Floor |
Selective Variable Variable Variable Variable N/A Selective Normal Normal Normal Normal |
>90% >90% >30% >90% >90% >90% >90% >30% >30% >30% - |
| Area, unit or department | Service illuminance
/ lux |
Max. point illuminance/ lux
(not to be exceeded) |
Unified
Glare Rating (UGR) |
Min.Ra | Measurement
Point |
Type of control | Standby lighting level (%) |
| Accident and emergency
- Admissions. reception - supplies stores - minor treatment area - minor operations
- couch (local) - general examination areas - procedure room
- resuscitation room
|
300 500 15000/30000
500 500 30000/60000
500
|
520 850 N/A
850 850 N/A
860
|
22 19 -
19 19 -
19
|
80 80 90
80 80 90
80 |
Work Surface Work Surface Adjustable to suit treatment area Over couch area Couch level Couch level Task illumination provided by minor treatment lamp Work Surface
|
Normal Normal Normal
Variable Variable Normal
Normal
|
>90% >30% >30%
>90% >90% >90%
>30%
|
| Audiology
- audio testing - consulting room - ear examination - vestibular testing (labyrinth) |
300 1000 (local) 100 |
520 n/a 170 |
19 - 19 |
80 90 80 |
Work Surface (examination lamp) Couch head and instruments
|
Variable -
|
>30% -
|
| Dentistry
- laboratories - reception/administration areas - surgeries/theatres - treatment rooms - white teeth matching |
300 8000 to 20000 500 5000 |
520 n/a 850 n/a |
19 - 19 - |
80 90 90 90 |
Work Surface Mouth Bench work surface Work Surface (TCP ≤ 6000 K)
|
Selective Variable Normal Normal |
>30% >90% >30% >30% |
| Diagnostics support
- aseptic laboratory - blood bank - colour inspection laboratory
- inspection - laboratories - laboratory (with computers) - pathology laboratory - relatives’ waiting room - seminar room - viewing/bier room
|
300 1000 (local)
500 (local) 500 300 500 300 300 30 to 150
|
520 n/a
n/a 850 520 850 520 520 n/a
|
19 -
- 19 19 19 19 19 19
|
80 90
80 80 80 80 80 80 80
|
Work Surface Bench (TCP ≤ 6500 K) Work Surface Bench Bench/desk Work Surface Bench Work Surface Work Surface Bier
|
Normal Normal
Normal Normal Normal Normal Variable Normal Variable
|
>90% >90%
50-90% 50-90% 50-90% 50-90% >30% >30% >30%
|
| Women’s services (maternity)
- applying sutures
- circulation space (day) - delivery - day - night - neonatal |
100 500 50 to 100 5 1000 (local) |
170 850 n/a 20 n/a |
19 19 19 - - |
80 80 80 80 80 |
Floor Work Surface Cot Cot Cot |
Normal Selective Normal Normal Normal
|
>30% >90% >90% >90% >90% |
| Mother and baby rooms
- circulation space (day) - day - night - nurseries (day) - nurseries (night) - milk kitchen - special care baby unit - teaching areas
|
50 to 100 5 100 5 300 1000 (local) 300
|
n/a 20 170 20 520 n/a 520
|
19 - 19 - 22 19 19
|
80 80 80 80 80 80 80
|
Cot Cot Floor Floor Bench Cot Bench/Work Surface
|
Normal Normal Normal Normal Normal Normal Normal
|
>90% >90% >30% >30% >30% >90% -
|
| Area, unit or department | Service illuminance
/ lux |
Max. point illuminance/ lux
(not to be exceeded) |
Unified
Glare Rating (UGR) |
Min.Ra | Measurement
Point |
Type of control | Standby lighting level (%) |
| General treatment areas
- autopsy (dissecting) table - autopsy rooms and mortuaries - dermatology
- dispensary - minor surgery/treatment - plaster room - resuscitation (general) - resuscitation/examination - pharmacy
|
500 (higher values could be required) 500 500 15000/30000 500 500 15000(local) 500
|
850 n/a
850 860 850 850 n/a 850
|
19 19
19 19 19 19 - 19
|
90 90
80 90 80 90 90 80 |
Work Surface (Local operating lamp)
Bench Work Surface Work Surface Head of trolley Work Surface
|
- -
Normal Normal Normal Normal Normal Normal
|
- -
>90% >90% >30% >90% >90% 50-90%
|
| Mortuaries and animal houses
- autoclave - body store - general - mortuary - operation - post mortem - staff change - store room - viewing room - waiting room
|
200 300 150 500 local 500 100 to 150 150 50/100 200 (min)
|
350 520 260 n/a 850 n/a 260 n/a n/a
|
19 19 19 - 19 19 19 19 19
|
80 80 80 90 90 80 80 80 80 |
Work Surface Work Surface Bier room Bench Work Surface/table Floor Work Surface Work Surface Floor
|
Normal Special Variable Normal Normal Normal Normal Variable Selective
|
>30% - >30% >30% >30% >30% - >30% >30%
|
| Engineering services
- ducts - plant room - roadways - workshop
|
300 7 300/500
|
520 12 n/a
|
22 - 22
|
80 80 80 |
Floor Road surface Bench
|
Normal - Normal
|
>90% - >30%
|
| Facilities support services
- laundry - linen store (Linen Department) - pack and dispatch - pressing - sewing room - wash and dry
|
100 300 300 500 (local) 300 |
170 520 520 n/a 520
|
19 19 19 19 19
|
80 80 80 80 80
|
Floor Bench Equipment Machine Equipment |
Normal Normal Normal Normal Normal
|
- >90% >90% >90% >90%
|
48.4.1. The lighting levels quoted above relate to the relevant task area. Levels of for the task areas and surrounding areas can be reduced where it can be justified by experienced staff or engineers. Lighting levels must, regardless, comply with the requirements of the National Building Regulations.
48.4.2. Lighting levels for external areas shall comply with the following table: (Adapted from CIBSE Lighting Guide 2: Hospitals and Healthcare settings).
Table 7 Levels of Indoor Lighting
| Area | Maintained average illuminance / lux | Maintained minimum illuminance / lux | Overall uniformity (not less than stated figure) | Threshold increment | Colour rendering (minimum) |
|
- monochrome - colour
|
0 -
20 30
15 20
|
5 15
8 12
6 12
|
0.4 0.4
0.4 0.4
0.4 0.4
|
≥10% ≥10%
|
≥60% ≥60%
≥20% ≥20%
≥20% ≥20%
|
49. Classification of Safety Services necessary for Medical Locations from SANS 10142-1
| Class | Response Time |
| Class 0 (No break) | Automatic supply available at no break
UPS backed up by Generator Required. |
| Class 0,15 (Very short break) | Automatic supply available within 0,15 s
UPS backed up by Generator Required. |
| Class 0,5 (Short break) | Automatic supply available within 0,5 s
UPS backed up by Generator Required. |
| Class 15 (Medium break) | Automatic supply available within 15 s
Generator Required |
| Class > 15 (Long break) | Automatic supply available in more than 15 s
Generator Required |
Note Safety Services in Medical locations are synonymous with Emergency Services.
50. Medical Location Classification
50.1. Group 0 location: where no applied part is intended to be used.
50.2. Group 1 location: Medical Location where applied parts are intended to be used.
- Externally, or
- To any part of the body, but not to the heart.
50.3. Group 2 location: Medical Location where applied parts are intended to be used in applications such as in an intracardiac procedure, in an operation (in an operating theatre) and in vital treatment where discontinuity (failure) of supply can cause danger to life.
Note: An intracardiac procedure is a procedure whereby an electrical conductor is placed within the cardiac zone of a patient or is likely to come into contact with the heart, such conductor being accessible outside the patient’s body. In this context, an electrical conductor includes insulated wires such as cardiac pacing electrodes or intracardiac ECG electrodes, or insulated tubes filled with conducting fluids.
50.4. For the allocation of medical location group and classification of safety service classes for medical locations see Table below as supplied in SANS 10142-1.
|
Medical Location |
Medical location group |
Safety service class | ||||
| 0 | 1 | 2 | ≥ 0,5 | 0,5
≤ 15 | ||
|
|
|
|
|
| ||
|
|
|
|
| |||
|
|
|
|
|
| ||
|
|
|
|
| |||
|
|
|
|
| |||
|
|
|
|
| |||
|
|
|
|
| |||
|
|
Radiology diagnostic and radio therapy room, other than mentioned under 21 |
X |
| |||
|
|
|
|
| |||
|
|
|
|
| |||
|
|
|
|
|
| ||
|
|
|
|
|
| ||
|
|
|
|
|
|
| |
|
|
|
|
|
|
| |
|
|
|
|
|
|
| |
|
|
|
|
|
| ||
|
|
|
|
|
| ||
|
|
|
|
|
| ||
|
|
|
|
| |||
|
|
|
|
| |||
|
|
|
|
| |||
|
|
|
|
|
| ||
|
a The room is not an operating theatre. |
||||||
50.5. In addition to the tables 0 and 50.4 above, generator supply is also required for:
- Night light in wards and ward corridors;
- All switched socket outlets used for patient life support anywhere in the facility;
- At least one patient lift or lift that can accommodate a bed for every 200 patients;
- Medical air compressor, vacuum pumps and gas alarm systems;
- Supply air fans serving theatres and uni-directional airflow systems;
- Isolation ward exhaust air fans.
- Mortuary Fridge Cabinets
- Nurse call System
- Fire detection system
51. General Requirements
51.1. Power supply to switched socket outlets in high care units, intensive care units and operating theatre units and recovery rooms must be on an earth monitoring system. Double pole isolators must be used for supply points in these areas and the power supply to these shall be fed from an isolation transformer.
51.2. Medical Location Group 1:
Switch Socket outlets in Medical Location 1 Shall have final Circuits for socket -outlets up to 16Amp shall be protected by earth leakage protection devices with a rated earth leakage tripping current ( rated residual current) not exceeding 30 Ma.
51.3. Medical Location Group 2:
Switch Socket outlets in Medical Location 2 the Medical Isolation Transformer (Medical IT) system shall be used for circuits that supply medical electrical equipment and systems intended for life support or surgical applications and other electrical Equipment located in the patient environment. In the case of each group 2 medical location, at least one separate medical IT system is necessary.
51.4. The Medical Isolation Transformer (MIT) shall be equipped with:
51.4.1. A 5 or 8 kVA Isolation Transformer complete with a 220 V Primary and 220 V / 110 V Secondary Winding with a centre Point Floating but bonded to the Earth monitor. The Secondary Side of the transformer shall provide 220 Volts between Line 1 and Line 2 (Note no Neutral with an Isolation Transformer) Line 1 and Line 2 will feed the Distribution Board for that particular Medical Group location, i.e. (Theatre No 1) or (ICU Bed 1-6) This local DB will then have a number of double pole Circuit Breakers feeding out to the outgoing Circuits feeding the Socket Outlets in the Medical Location 2 Area. Note that at least two circuits are required to each ICU Bed or Theatre Panel, and Theatre Pendant. Also note that all Switch Socket Outlets in a Medical Location 2 Area have to be double Pole Switched via a double pole Isolator (Provided two circuits provided) or a double pole Circuit Breaker.
51.4.2. The Transformer shall be installed either in a cabinet/DB or enclosure, to prevent unintentional contact with live parts. The Transformer / DB should be located close to the Group 2 Medical Location but consideration should be given to providing the DB outside the red line area of both the Theatres and ICU Areas, so maintenance can be carried out without the need to be gowned up. Line 1 & 2 and Earth should all be Insulated wires with the colour of Line 1 & 2 being different from red and black suggest Brown and Blue wire is used for Line 1 & 2 and green for Earth (Note this Earth wire should be connected to an insulated Earth bar dedicated to that particular Group two location and bonded to the centre point of the secondary winding.) Note a Separate Dirty earth (Equipotential bonding) should also be provided to the metal work of the Plugs, Theatre Panel, and Pendant this earth shall be connected to the Main Building Earth.
51.4.2. An insulation -monitoring device that:
- Has an internal impedance of at least 100 k Ohm;
- Has a test voltage not exceeding 25 V DC.
- Is of a current, even under fault conditions, not exceeding 1 mA DC. and
- Shall indicate, at least when the insulation resistance has decreased to 5 k Ohm.
A test device shall be provided to test this facility to ensure that the alarm (Audible and visual) operates when the insulation resistance reaches 5 k Ohm;
To test the System two male plugs should be used each with a resistor of 5 k Ohm. Plug No 1 should have a 5 k Ohm resistor bridged from the Earth Pin to the Right Hand live Pin. Plug No 2 should have a 5 k Ohm resistor bridged from the Earth Pin to the Left Hand live Pin.
51.4.4. Medical Isolation Transformer Alarm.
For each Medical IT system an audible and visual alarm shall be provided in the Theatre Area a alarm shall be provided on theatre Panel and repeated back to the main Nurse Station in Theatre Area .The Alarm shall consist of Green Light indicating Healthy, a red light indicating fault, a audible Alarm to also indicate fault and a local audible alarm mute button. The Visual signal shall revert to green and the audible alarm shall be automatically reset on the removal of the fault condition
Table 4 – Required for Medical Isolation Transformers (MIT) and Switch Socket Outlets (SSO)
| Description | Medical Location Group | Number and type of Switch Socket Outlets (SSO) | ||||||
| Location on wall or from UPS on wall or Power Skirting | Dedicated Red SSO fed from UPS on wall or Power Skirting | Hospital Service Panel | ||||||
| Bed Head Trunking Backed up by Standby | Bed Head Trunking Fed from MIT and UPS. 16 A Red Dedicated SSO with Blue DPS[1] | Theatre Panel Fed from MIT and UPS Red Dedicated SSO w Blue DPS | Pendant Panel Fed from MIT and UPS Red Dedicated SSO w Blue DPS | On Wall Fed from MIT and UPS Red Dedicated SSO with Blue DPS | ||||
| Office Station | 0 | 1 x Red 16A Normal | 1 x Red 16A Dedicated | |||||
| Laboratory Work Station | 0 | 1 x Red 16A Normal | 1 x Red 16A Dedicated | |||||
| Ward Office | 0 | 1 x Red 16A Normal | 1 x Red 16A Dedicated | |||||
| Ward Nurse Station | 0 | 1 x Red 16A Normal | 1 x Red 16A Dedicated | |||||
| Ward | 1 | 1 x 16A Normal / Ward for Cleaning + 1 x 16 Amp in ceiling for TV Point/ Bed(if required). | 3x 16A per bed | |||||
| Ward Kitchen | 0 | 2 x 16A Normal on Wall at 1200 mm over counter + 1 x 16 A next to Sink or Hydro Boil. | 1 x 16A supply for Fridge on generator supply | |||||
| Ward Corridor | 0 | 1 x 16A Normal / Every 15 m of corridor for Cleaning | 1 x Red 16A Dedicated for Crash Cart Position. | |||||
| Ward Staff Rest Room | 1 | I x 16 A on Wall for Cleaning, 1 x 16 A above counter for Electrical Appliances + 1 X 16 A next to sink for Hydro boil | ||||||
[1] Note: DPS is an abbreviation for double pole switch
| Description | Medical Location Group | Number and type of Switch Socket Outlets (SSO) | ||||||
| Location on wall or from UPS on wall or Power Skirting | Dedicated Red SSO fed from UPS on wall or Power Skirting | Hospital Service Panel | ||||||
| Bed Head Trunking Backed up by Standby | Bed Head Trunking Fed from MIT and UPS. 16 A Red Dedicated SSO with Blue DPS[1] | Theatre Panel Fed from MIT and UPS Red Dedicated SSO w Blue DPS | Pendant Panel Fed from MIT and UPS Red Dedicated SSO w Blue DPS | On Wall Fed from MIT and UPS Red Dedicated SSO with Blue DPS | ||||
| Theatre Equipment Room | 0 | 15 x 16A Normal on Wall at 1200 mm over shelf | ||||||
| Theatre Post Op | 2 | 6 x 16 A Dedicated SSO per Bed | ||||||
| Operating Theatre | 2 | 8 x 16 A Dedicated SSO per Pendant | 8 x 16 A Dedicated SSO per pendant | 4 x 16 A Dedicated SSO | ||||
| Cath Lab Operating Room | 2 | 4 x 16 A Dedicated SSO | ||||||
| Cath Lab Control Room | 0 | 1 x 16A Normal per Station | 1 x Red 16A Dedicated per Station | |||||
| Cath Lab Equipment Room:
160 kva dedicated UPS Required to feed Dedicated DB and Equipment |
||||||||
| Autoclave | In autoclave plant room. 3-Phase 380V, 80A per autoclave | |||||||
| Instrument Washer | In CSSD. Typically 3-Phase 380V, 15A per washer | |||||||
| Theatre Corridor | 1 | 1 x 16A Normal for every 15 m of corridor for Cleaning | 1 x Red 16A Dedicated for Crash Cart Position | |||||
| Description | Medical Location Group | Number and type of Switch Socket Outlets (SSO) | ||||||
| Location on wall or from UPS on wall or Power Skirting | Dedicated Red SSO fed from UPS on wall or Power Skirting | Hospital Service Panel | ||||||
| Bed Head Trunking Backed up by Standby | Bed Head Trunking Fed from MIT and UPS. 16 A Red Dedicated SSO with Blue DPS[1] | Theatre Panel Fed from MIT and UPS Red Dedicated SSO w Blue DPS | Pendant Panel Fed from MIT and UPS Red Dedicated SSO w Blue DPS | On Wall Fed from MIT and UPS Red Dedicated SSO with Blue DPS | ||||
| ICU Circulation Space | 1 | 1 x 16A Normal per 25m2 for cleaning | 1 x Red 16A Dedicated for Crash Cart Position. | |||||
| Neo Natal ICU Cots Note: Care should be taken when sizing the Isolating Transformers to include the Heating Load | 2 | 15 x 16A Dedicated per Bed on the same Isolating Transformer but two separate Circuits | 15 x 16A Dedicated per Bed on the same Isolating Transformer but two separate Circuits (Note if you are using a 8 kva Isolating Transformer you can put 6 Beds on one Transformer) | |||||
| High Care | 2 | 15 x 16A Dedicated per Bed on the same Isolating Transformer but two separate Circuits (Note if you are using a 8 kva Isolating Transformer you can put 6 Beds on one Transformer) | 15 x 16A Dedicated per Bed on the same Isolating Transformer but two separate Circuits (Note if you are using a 8 kva Isolating Transformer you can put 6 Beds on one Transformer) | |||||
| High Care Nurse Station (per workstation) | 1 | 1 x 16A Normal per Station | 2 x Red 16A Dedicated for Crash Cart Position | |||||
| ICU and Ward Equipment Room | 0 | 15 x 16A Normal on Wall at 1200 mm over shelf | ||||||
| Description | Medical Location Group | Number and type of Switch Socket Outlets (SSO) | ||||||
| Location on wall or from UPS on wall or Power Skirting | Dedicated Red SSO fed from UPS on wall or Power Skirting | Hospital Service Panel | ||||||
| Bed Head Trunking Backed up by Standby | Bed Head Trunking Fed from MIT and UPS. 16 A Red Dedicated SSO with Blue DPS[1] | Theatre Panel Fed from MIT and UPS Red Dedicated SSO w Blue DPS | Pendant Panel Fed from MIT and UPS Red Dedicated SSO w Blue DPS | On Wall Fed from MIT and UPS Red Dedicated SSO with Blue DPS | ||||
| Casualty Treatment Rooms 1 | 1 | 1 x 16A Normal per Ward for Cleaning + 1 x 16 Amp in ceiling for TV Point per Bed. 2 x 16A per Bed | ||||||
| Procedure Rooms 1 | 1 | 1 x 16A Normal per Ward for Cleaning | 4 x 16A per Bed per two Circuits. | |||||
| Casualty Ward Corridor 0 | 0 | 1 x 16A Normal per every 15 m of corridor for Cleaning | 1 x Red 16A Dedicated for Radiology Procedure Crash Cart Position | |||||
| Rooms Dedicated 125 Amp Supply to dedicated | 1 | 1 x 16A Normal | 1 x Red 16A Dedicated | |||||
| Radiology Control Room | 0 | 1 x 16A Normal per Station | 1 x 16A Normal per Station | |||||
| Maternity Delivery Rooms | 2 | 4 x 16A Dedicated per Bed on the same Isolating Transformer but two separate Circuits (Note if you are using a 8 kva Isolating Transformer you can put 6 Beds on one Transformer) | ||||||
| Description | Medical Location Group | Number and type of Switch Socket Outlets (SSO) | ||||||
| Location on wall or from UPS on wall or Power Skirting | Dedicated Red SSO fed from UPS on wall or Power Skirting | Hospital Service Panel | ||||||
| Bed Head Trunking Backed up by Standby | Bed Head Trunking Fed from MIT and UPS. 16 A Red Dedicated SSO with Blue DPS[1] | Theatre Panel Fed from MIT and UPS Red Dedicated SSO w Blue DPS | Pendant Panel Fed from MIT and UPS Red Dedicated SSO w Blue DPS | On Wall Fed from MIT and UPS Red Dedicated SSO with Blue DPS | ||||
| Dialysis Treatment Beds.
Note that the Equipment can include Water Heating with high kw loading so care should be taken when sizing the Isolating Transformers |
2 | 4 x 16A Dedicated per Bed on the same Isolating Transformer but two separate Circuits | ||||||
51.5. Uninterrupted Power System must be provided for operating theatre luminaries and all life support systems and computer systems where a break in electrical supply cannot be tolerated. The whole installation must conform to SANS 1474 of 1988.
51.6. Socket outlets for Dialysis units, in close proximity to water points or drains, shall be of the totally waterproof IP65 type, which also seal water-tight when the socket is removed.
51.7. Where more than one electrical transformer is used, they should preferably be located in separate structural enclosures. This is to prevent potential damage to an adjacent transformer if one is damaged.
51.8. All distribution boards fed from normal mains supply shall be painted Electric Orange, colour B26 to SABS 1091.
51.8. All distribution boards fed from standby emergency power supply shall be painted Signal Red, colour A11 to SABS 1091.
51.9. All distribution boards fed from UPS power supply shall be painted Blue colour F06 to SABS 1091.
51.10. All cable transition boxes shall be painted the appropriate colour corresponding to the source of the power supply.
51.11. All cables installed on surface mounted cable ladders shall be of the PVC/PVC/SWA/ECC/PVC type to SANS 101507 rated at 600/1000 Volt.
51.12. Electrical circuits to be engraved on base 3mm lettering indicating circuit number and DB.
51.13. Electrical Certificate of Compliance.

Electronic installations
52. The design parameters for internal spaces should be found in the detailed room requirement sheets published in the individual IUSS guidance documents of the various functional units. Where these room requirement sheets are absent or lacking adequate information, the data contained in this document may be used.
53. Nurse call system with emergency (nurse assistance) and TV control handsets (Interchangeable with LED PEAR PUSH).
The nurse system enables the patient to call a nurse for assistance from his bed or from a bath, shower and toilet. The system also enables the staff to call for assistance (EMERGENCY CALL) from any bed and treatment room etc.
- When a patient nurse call or staff emergency call is enabled the system must produce an intermittent AUDIBLE chimes or bleeper tone at the nurses’ station or/and duty room. Three different sounding tones must be produced for normal Patient call, Bathroom call and Emergency (nurse assistance) call.
- The system must also provide a VISUAL indication, at the nurse station (LED Mimic Panel and/ or Computer Monitor or LCD Display Panel), above the door to the passage of the activated unit, and at the actual activated unit (reassurance LED).
- The system must be so designed that any call may ONLY be RESET at the point of origin.
- The system must automatically activate a nurse call when the Hand Held Unit (Handset) or Pear Push unit is accidently pulled out from the Bed Head Unit.
- The Bed Head Unit must be compatible with Hand Held Unit (with TV Control), Rehab Hand Held Unit & Pear Push. (Inter-changeable)
- A Central Monitoring PC, or PC per duty station replacing Mimic Panel, must keep records of all events. (Optional)
- The system must be purpose made and aesthetically pleasing with components (call & reset units etc) manufactured from matching injection moulded ABS plastic. A system made up of push buttons etc mounted directly onto standard electrical plates will not be accepted.
54. Automatic fire detection in Hospitals
The Fire Detection System shall comply with SANS 10400 SANS 10139 & SANS 322. The Fire Detection System must be provided throughout the Facility and be a Addressable Fire Detection System, Note that Audible Fire Alarms which could panic the patients should not be provided instead Visual Strobe Lights should be provided at all Nurse stations, Reception and Security Office.
Audible alarms can be used in noisy areas such as plant rooms.
The wiring for the Automatic Fire Detection System shall be KAL21B Fire Alarm cable, or equivalent 2 hour rated cable (1,5mm² minimum cross Sectional area)
55. Public Address and Evacuation in Hospital
The Public Address and Evacuation System shall comply with EN54 and provide voice message via fire retardant speakers throughout the hospital Circulation Areas, Staff Areas, Public Toilets. The wiring for the Evacuation Speakers shall be KAL21B Fire Alarm cable, or equivalent 2 hour rated cable (1,5mm² minimum cross Sectional area). As part of the Hospital Design the Hospital should be zoned to allow Evacuation of Individual zones in the event of a fire or other Emergency.
Wet Services
56. Plumbing services (Water supply and drainage) must comply as a minimum with the following Standard Specifications and Codes of Practice:
- SANS 10400: The Application of the National Building Regulations, including Part XA: Energy Use in Buildings
- SANS 10252 – Part 1 – Water Supply Installations for Buildings
- SANS 10252 – Part 2 – Drainage Installations for Buildings
- UK Department of Health Technical Memorandum 04-01: The Control of Legionella, hygiene, “safe” hot water, cold water and drinking water systems: Part A: Design, Installation and Testing, and Part B: Operational Management or the equivalent SANS standard when available.
57. Where any apparent conflict between the functional requirements and the regulatory “deemed to satisfy” guidance emerges, the rational design route to regulatory compliance would need to be followed so as not to compromise any system’s functional compliance.
58. Cold water storage, dedicated to the domestic water requirements of the facility, must be provided on the site. A minimum usable volume of 500 litres per bed must be provided.
59. If water storage is required for fire protection purposes (Sprinklers, Fire Hydrant supply) it must be stored separately from domestic water, unless adequate provision for the prevention of stagnation of the fire service reserve within the tank can be made.
60. Tanks must be accessible both on the outside as well as the inside, and provision for cleaning the tanks without interrupting water supply to the hospital must be made. Access manholes must be lockable, and a facility for draining the tank or individual compartments within it, must be made.
61. All openings to the tank (Overflows, vent pipes, etc) must be provided with screens.
62. Underground tanks, with their inherent risk of contamination must be avoided at all cost. If unavoidable, the tank should be constructed in a water tight bund allowing sufficient space all round for inspection and maintenance, and a sump for collecting drainage water
63. Cold water storage tanks must be located such that heat gain to the water is minimised. Cold water storage temperatures 20 C and lower will prevent the colonisation by or multiplication of Legionella
64. Site water reticulation must be designed using sound engineering principles, with adequate provision for isolating sections of the reticulation whilst keeping the remainder in operation being made.
65. Fire protection water reticulations must be kept totally separate from the domestic water reticulation
66. Water distribution may be gravity fed, or alternatively supplied via booster pumps. Pumps must be suited to handling potable water, and provision for built in redundancy must be made. Booster pumps must be supplied off standby power.
67. Hot water supply temperature to general patient care and staff areas must be controlled at 55 C, and must not exceed 60°C, except during a sanitation cycle as described hereunder.
68. Hot water generation systems must where possible use waste heat recovery from a central air conditioning system, if employed.
69. The facility for thermal disinfection of the hot water storage and circulation system must be provided in the system design. This can take the form of controlled heating of the storage vessel and circulating mains to 60°C during periods of low water and power demand. The use of shunt pumps to circulate hot water from the top level to the lowest level of the hot water tank during the sanitation cycle must be considered.
70. Hot water supply to paediatric wards, as well as to geriatric and to neonatal bathing facilities shall not exceed 42°C at the point of supply. If thermostatic mixing valves are employed to achieve this, they must be fitted with a safety feature such that the water flow is cut off within 2 seconds of the cold water supply to the valve being interrupted. The valve must also be able to control the set temperature with a pressure ratio of incoming hot to cold water supply pressure of up to 10 to 1.
71. Toilet flushing systems must be provided with easily identifiable dual flush mechanisms, one being for low flush water flow, the other for standard flush water flow.
72. All sanitary fittings must be piped such that the hot water control is on the left hand side, and the cold water supply is on the right hand side when facing the fitting.
73. Branch pipes (dead legs) between water heating equipment or hot water circulating mains and sanitary fittings must be sized and located such that the maximum waiting time for hot water to emerge from the fitting does not exceed 12 seconds.
74. Mixing taps in patient care areas must be elbow action type, installed such that the tap can be opened and shut by means of simple elbow action.
75. The discharge from kitchen floor drains and other kitchen drain points such as sinks, dishwashing washing, machines, and cooking equipment wash down, likely to contain grease, must be taken via a separate discharge system to a suitable grease interceptor, installed in a position to allow easy access for removal of intercepted grease and oil.
76. The drain pipes from equipment likely to produce high temperature discharge, such as autoclaves, sterilisers and cooking equipment must be from materials able to withstand such temperatures, installed such that distortion and/or expansion can be accommodated by the system.
77. Vertical pipe runs (Drainage stacks and water supply mains) in multi storey buildings must be housed in continuous vertical service ducts with easy access from non-patient areas at each level.
78. Condensate drains from air conditioning and refrigeration systems must discharge into piped drainage systems.
79. Anti-Backflow protection devices shall be fitted to faucets with hand-held shower heads to prevent back siphoning should the supply water pressure fail.
80. In areas housing patients at unusual risk of infection, faucets should not be fitted with low-flow or aerating devices which may increase the rate of aerosolisation of infectious droplets.
81. Legionella Control
- A facility-wide legionella control plan shall be in place which will inform operation, maintenance and design of water systems.
- This Plan must include a Legionella risk assessment document, listing all areas where the bacteria may occur. This must address specifically air conditioning condenser water systems, domestic hot and cold water installations, irrigation water storage and distribution systems, etc.
- The Plan must refer to as-built drawings identifying positions and layouts of plant and installations liable to cause a risk of Legionella being generated
- The facility’s Maintenance Procedures must describe all measures to be taken to minimise proliferation of Legionella. This is to include procedures and frequency of sanitation/disinfection, purging of dead legs on circulating systems, sample taking and testing at specific intervals, as well as a responsibility matrix of personnel
For additional information refer to the Legionella Control article.
Lifts
82. Standards and Regulations Pertaining to Lifts and Lifting Operations:
- SANS 50081 - SAFETY RULES FOR THE CONSTRUCTION AND INSTALLATION OF LIFTS - PARTICULAR APPLICATIONS FOR PASSENGER AND GOODS LIFTS
- SANS 10400 –SS3 FACILITIES FOR DISABLED PERSONS: LIFTS
- SANS 10400 –TT45 FIRE PROTECTION: LIFT SHAFTS
- SANS 10400 –TT46 FIRE PROTECTION: LIFT
- SANS 10400 –TT47 FIRE PROTECTION: FIREMAN’S LIFT
- SANS 10400 –TT48 FIRE PROTECTION: STRETCHER LIFT
83. Planning for circulation, capacity and location of lifts
- A lift traffic plan should be developed. Detailed lift traffic planning is beyond the scope of this document. A specialist advisor should be consulted to assist in the planning of lifts within the general principles of lifts services for healthcare buildings.
- General Lift Planning Principles
- The operational details of the facility must be understood for effective lift planning. These include:
- Number of visitors and visiting hours
- Number of staff, shift hours and ward round schedules
- Numbers of day and overnight patients
- Increased provisions for reduced mobility persons
- Housekeeping schedule
- Evacuation plan for reduced mobility patients.
- Whenever possible, lifts should be provided in pairs to allow for continued operation during maintenance and breakdown.
84. TYPES OF LIFTS:
84.1. PASSENGER LIFTS
- These lifts shall be able to accommodate general passenger traffic including ambulatory and semi ambulatory passengers. It shall be able to accommodate reduced mobility passengers using mobility aids and wheelchairs. Refer to SANS 50081-70, Table 1
- The entrance to a passenger lift shall not be less than 1100 mm in width.
- The depth of a passenger lift shall not be less than 1400mm deep.
- Passenger lifts shall have a mirror on the top half of the rear wall equal to the width of the lift to enable wheelchair users to back out of the lift where the lift has internal dimensions less than 1,5 m in width and 2,0 m in depth
- At least on one side wall of the car a handrail shall be installed
84.2. BED LIFTS
- Bed lifts shall have internal dimensions of 1 800 mm wide by 2 700 mm deep to accommodate most beds with staff and medical equipment.
- The entrance to a bed lift shall not be less than 1370 mm in width.
- The power supply to the motor operating such a lift shall be able to withstand fire for at least 120 min.
84.3. STRETCHER LIFTS
- Stretcher lifts shall have internal dimensions of 1 400 mm wide by 2 400 mm deep to accommodate most trollies or stretchers.
- The entrance to a stretcher lift shall not be less than 1370 mm in width.
- The power supply to the motor operating such a lift shall be able to withstand fire for at least 120 min.
84.4. GOODS LIFTS
- Goods lifts are for the movement of conventional goods and items that could not reasonably use passenger lifts without causing discomfort to passengers or damage and soiling of the lift car.
- Goods lifts can be designed to accommodate passengers.
84.5. SERVICE LIFTS
- Service lifts are not designed for accommodate passengers. They are typically of the “dumb waiter” style dispatched between service hatches.
84.6. HOUSEKEEPING LIFTS
- Housekeeping lifts are similar in function to goods lifts but are intended for the movement of cleaning supplies, medical supplies and equipment, linen etc.
| Load: | Speed: | Car size:
W mm x D mm |
Door type: | Door opening:
W mm x H mm |
Shaft size: minimum
W mm x D mm |
Pit depth:
mm |
Headroom: mm |
|---|---|---|---|---|---|---|---|
| 630 kg / 8 persons | 1.0 m/s
1.0 m/s 1.75 m/s |
1100 x 1400 | CLD
|
800 x 2100
|
1800 x 1800
|
1450
1500 1600 |
4200
4300 4400 |
| 800 kg / 10 persons | 1.0 m/s
1.0 m/s 1.75 m/s |
1350 x 1400 | CLD
|
800 x 2100
|
2000 x 1800
|
1450
1500 1600 |
4200
4300 4400 |
| 1000 kg / 13 persons | 1.0 m/s
1.0 m/s 1.75 m/s |
1600 x 1400 | CLD
|
1000 x 2100
|
2300 x 1800
|
1450
1500 1600 |
4200
4300 4400 |
| 1250 kg / 16 persons | 1.0 m/s
1.0 m/s 1.75 m/s |
2000 x 1400 | CLD
|
1000 x 2100
|
2700 x 2000
|
1450
1500 1600 |
4200
4300 4400 |
| Load: | Speed: | Car size:
W mm x D mm |
Door type: | Door opening:
W mm x H mm |
Shaft size: minimum
W mm x D mm |
Pit depth:
mm |
Headroom: mm |
|---|---|---|---|---|---|---|---|
| 1000 kg / 13 persons | 1.0 m/s
1.0 m/s 1.75 m/s |
1100 x 2100 | CLD
|
1000 x 2100
|
200 x 2600
|
1450
1500 1600 |
4200
4300 4400 |
| 1600 kg / 21 persons | 1.0 m/s
1.0 m/s 1.75 m/s |
1400 x 2400 | CLD
TLD CTLD |
1300 x 2100
1300 x 2100 1400 x 2100 |
2800 x 2800
2500 x 2900 2400 x 2900 |
1450
1500 1600 |
4200
4300 4400 |
| 2000 kg / 26 persons | 1.0 m/s
1.0 m/s 1.75 m/s |
1500 x 2700 | CLD
TLD CTLD |
1300 x 2100
1300 x 2100 1400 x 2100 |
2800 x 3100
2600 x 3150 2450 x 3150 |
1450
1500 1600 |
4200
4300 4400 |
| 2500 kg / 33 persons | 1.0 m/s
1.0 m/s 1.75 m/s |
1800 x 2700 | CTLD
|
1400 x 2100
|
2900 x 3150
|
1450
1500 1600 |
4200
4300 4400 |
PART D - COMMISSIONING AND HANDOVER
Deliverables
1. This section is intended to detail the commissioning deliverables required before handover of building engineering services for operation. For further detail on commissioning and handover the IUSS Commissioning Health Facilities guidance document should be referred to.
2. Project Close-out deliverables include:
- Final Works completion lists
- Financial reports and final accounts
- Facilitation in development of Operation and Maintenance Manuals (O&Ms), warranties and guarantees.
- Approved As-Built Drawings
- Electrical Certificates of Compliance
3. Maintenance manuals shall be timeously issued and shall include:
3.1. Designer and installer contact information
3.2. System information
- System description
- Suppliers list
- Equipment List
- Equipment data sheets
- Materials of construction data sheets
- Warranty information
3.3. Operational parameters
- Start up and shut down procedures
- Special instructions
- Security and access details
- Fault finding procedures
- Alarm management and data logging
3.4. Validation and commissioning
- Approved reports and data
- Relevant test protocols
- Relevant test plans
- Installed and test equipment calibration certificates
- Commissioning certificates
- Beneficial Occupation and Handover certificates
3.5. Spare parts list
3.6. Electronic Data Backup (Read only Media)
3.7. Approved “As-Built” Drawings
- Process diagrams
- Wiring Diagrams
- Control Diagrams
- System Plans
- Training records
3.8. Training Records
3.9. Training Materials
Commissioning of ventilation systems
4. Commissioning of ventilation and air conditioning systems shall comprise the following:
- Confirmation of accuracy of measurements.
Measurement accuracy depends on equipment accuracy and repeatability. Factors that would impact on the accuracy of measurement include:
- Operator error and competence
- Type and quality of measuring device
- Quality and adherence to measurement protocols.
4.2. Proof of competence of commissioning technician or engineer
4.3. Commissioning method statements or protocols shall be developed, recorded and adhered to, to ensure all technicians work to the same procedures and sequences. In some instances, such as healthcare units where the ventilation system is critical to that unit’s clinical outcomes or to the safety of occupants, the client or client’s representative may request that these method statements be issued for formal approval before commencement of commissioning.
4.4. As the operational parameters of variable air volume systems are more complex that constant volume systems, the designer is to provide details of all relevant aspects of these systems such that the commissioning specialist can sufficiently develop an appropriate plan the commissioning.
4.5. Preliminary inspections should be completed before the systems are started up for commissioning. Typically these inspections should include:
- The state of completion of the building and the condition of details such as openable windows, doors and ceilings.
- Building cleanliness as it pertains to the ventilated spaces as well as the equipment plant rooms.
- Ducting and ventilation components should be inspected internally and externally for system cleanliness. Prior to fitting filters the following components should be checked for completion, correctness and cleanliness:
- Air intakes screens and mixing plenums
- Heating components
- Cooling components
- Condensate and drip trays
- In duct UVGI systems
- Humidifiers
- Fan and equipment chambers including safeties and interlocks
- Sensors and gauges
- Airflow controllers and fire damper
- Filter frames and orientation thereof
- Insulation
- Ducting and air terminals
- Electrical Equipment should be inspected for completion, correctness, labelling and cleanliness. Prior to running any electrical rotating or control equipment the following check should be completed.
- Local isolators of motors, electric heaters and control circuits including labelling.
- Electrical safety
- Motor starters and frequency drives set correctly for overload and motor restart ratings.
- Direction of rotation of motors on motor shafts
- Motor starting current and sequencing
4.6. An initial running-in period should be conducted at low load before the installation of the filters. This running period is to ensure flushing of ducting, and allow checking of the system operation. During the this period the system should be shut down and restarted to ensure that the controls, fuses and switchgear function correctly; however, repeated rapid restarts should be avoided as this can over-stress the control gear and fuses.
4.7 After the initial running-in the filters can be installed by a suitably qualified technician and the system should then be run at normal load. New filters should be installed before the final proportional balancing commences.
4.8. The proportional balancing of the airflow should be delayed until the ventilation system has been run-in under normal load for a few days to ensure stability of the system. The airflow balancing should be conducted in accordance with good engineering principles such as those described in SANS 10173, the ASHRAE Fundamentals Handbook, CIBSE Commissioning Code A or BSRIA Application Guide 3/89.1 depending on the system requirements.
4.9. For variable air volume systems, the commissioning tests should demonstrate system performance across the design diversity.
4.10. A definitive total airflow measurement should be taken in either a section of the main duct, where duct length and turbulence allow, or in the branch ducts. This value shall be recorded, compared against the design values and tolerances and reported on in the commissioning reports including the percentage of the design flow rates.
4.11. The final airflow measurements shall be taken at all air terminals (supply, return and exhaust) using airflow capture hoods where the terminal generates turbulence and these values shall be recorded, compared to design values and tolerances and reported on in commissioning reports including the percentage of the design flow rates.
4.12. Direction, drop and throw of air terminals shall be assessed by the responsible engineer to confirm the correct air distribution within ventilated spaces.
4.13. The minimum outside air portion should be demonstrated and recorded across the system’s operational diversity.
4.14. A condition of system acceptance is that the commissioning tests be witnessed before signing off. This process could involve the repetition of only a selection of the tests under the observation of an authorised witness or responsible engineer. The following aspects should be demonstrated:
- Performance of the system according to the overall design requirements within specified limits
- Repeatability of performance and measurement results
PART E - EXAMPLES
Mechanical system configurations
1. HOT WATER GENERATION SYSTEM
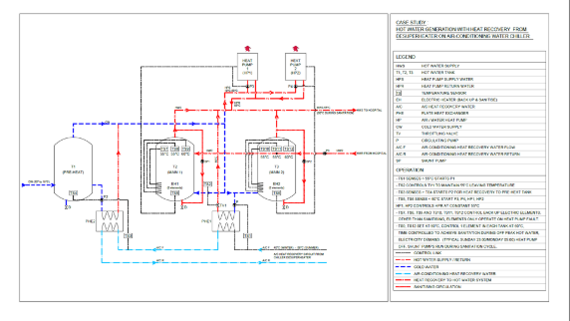
2. THEATRE VENTILATION SYSTEMS
The following examples indicate typical system configurations schematically.
2.1 UDAF Recirculation

2.2. UDAF Full Fresh Air & Exhausted

2.3. Major Theatre: Recirculation
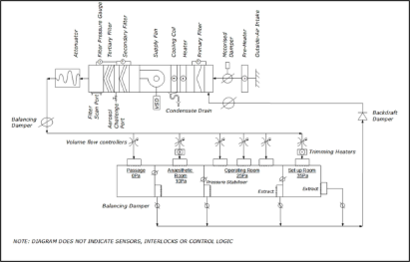
2.4. Major Theatre: Full Fresh Air Supply only

2.5. Minor Theatre: Recirculation

2.6. Minor Theatre: Full Fresh Air & Exhausted

2.7. Minor Theatre: Full Fresh Air Supply only

3. AIRBORNE PRECAUTION ROOMS AND THEATRES
3.1 Energy Recovery Systems for Airborne Precaution Rooms

4. Energy Recovery Systems for Airborne Precaution Theatres

REFERENCES
Applicable Regulations and Standards:
National Health Act 2004. (61 2003). Cape Town South Africa: Government Gazette.
Ammended Occupational Health and Safety Act 2004. (181 1993) South Africa: Department of Labour.
South African Bureau of Standards, 2009. SANS 10142-1:2008 The wiring of premises Part 1: Low-voltage installations. Pretoria South Africa: SABS Standards Division.
South African Bureau of Standards, 2003. SANS 10173:2003 The installation, testing and balancing of air-conditioning ductwork. Pretoria South Africa: SABS Standards Division.
South African Bureau of Standards, 2012. SANS 10252-1:2012 Water supply and drainage for buildings Part 1: Water supply installations for buildings. Pretoria South Africa: SABS Standards Division.
South African Bureau of Standards, 1993. SANS 10252-2:1993 Water supply and drainage for buildings Part 2: Drainage installations for buildings. Pretoria South Africa: SABS Standards Division.
South African Bureau of Standards, 1999. SANS 10313: 1999 Protection of structures against lightning. Pretoria South Africa: SABS Standards Division.
South African Bureau of Standards, 1990. SANS 10400-2: 1999 Code of Practice for The Application of the National Building Regulations. Pretoria South Africa: SABS Standards Division.
South African Bureau of Standards, 2005. SANS 1067-1:2005 Copper-based fittings for copper tubes Part 1: Compression fittings. Pretoria South Africa: SABS Standards Division.
South African Bureau of Standards, 2005. SANS 1067-2:2005 Copper-based fittings for copper tubes Part 2: Capillary solder fittings. Pretoria South Africa: SABS Standards Division.
South African Bureau of Standards, 2012. SANS 1091:2012 National colour standard. Pretoria South Africa: SABS Standards Division.
South African Bureau of Standards, 2005. SANS 1238:2005 Air-conditioning ductwork. Pretoria South Africa: SABS Standards Division.
South African Bureau of Standards, 2008. SANS 1409:2008 Outlet sockets and probes for medical (gas and vacuum) services used in hospitals. Pretoria South Africa: SABS Standards Division.
South African Bureau of Standards, 2008. SANS 1424:2008 Filters for use in air-conditioning and general ventilation. Pretoria South Africa: SABS Standards Division.
South African Bureau of Standards, 2011. SANS 1453:2011 Copper Tubes for Medical Gas and Vacuum systems. Pretoria South Africa: SABS Standards Division.
South African Bureau of Standards, 1999. SANS 14644-1:1999 Cleanrooms and associated controlled environments - Part 1: Classification of air cleanliness. Pretoria South Africa: SABS Standards Division.
South African Bureau of Standards, 2003. SANS 14644-2:2003 Cleanrooms and associated controlled environments - Part 2: Specifications for testing and monitoring to prove continued compliance with ISO 14644-1. Pretoria South Africa: SABS Standards Division.
South African Bureau of Standards, 2003. SANS 14644-4:2003 Cleanrooms and associated controlled environments - Part 4: Design, construction and start-up. Pretoria South Africa: SABS Standards Division.
South African Bureau of Standards, 1988. SANS 1474: 1988 Uninterruptible Power Supplies. Pretoria South Africa: SABS Standards Division.
South African Bureau of Standards, 2009. SANS 7396-1:2009 Medical gas pipeline systems Part 1: Pipeline systems for compressed medical gases and vacuum. Pretoria South Africa: SABS Standards Division.
South African Bureau of Standards, 2008. SANS 7396-2:2008 Medical gas pipeline systems Part 2: Part 2: Anaesthetic gas scavenging disposal systems. Pretoria South Africa: SABS Standards Division.
- All local Municipal laws and regulations,
- ISO 14644-3:, Cleanrooms and associated controlled environments - Part 3: Test Methods Australasian Health Infrastructure Alliance,2006. Australasian Health Facility guidelines [online] Available at: http://healthfacilityguidelines.com/guidelines.htm [Accesed ...].
- ISO/DIS 5359. Anaesthetic and respiratory equipment - Low-pressure hose assemblies for use with medical gases,
- National Health Act, 2004 (Act No. 61 of 2003).
- Occupational Health and Safety Act, of 1993
- Regulations of the Local Electricity Authority,
- SANS 10114: Lighting Requirements,
- SANS 10142-1: The wiring of premises Part 1: Low-voltage installations,
- SANS 10173: The installation, testing and balancing of air-conditioning ductwork,
- SANS 10224: Non-flammable medical gas pipeline,
- SANS 10252-1: Water supply and drainage for buildings Part 1: Water supply installations for buildings,
- SANS 10252-2: Water supply and drainage for buildings Part 2: Drainage installations for buildings,
- SANS 10313: 1999 Protection of structures against lightning,
- SANS 10400: Code of Practice for The Application of the National Building Regulations,
- SANS 1067: Copper-based fittings for copper tubes Part 1: Compression fittings,
- SANS 1067: Copper-based fittings for copper tubes Part 2: Capillary solder fittings,
- SANS 1091: Colour Coding of Services,
- SANS 1140: Identification colour marking Part 4: Contents of taps and valves in laboratories,
- SANS 1238: Air-conditioning ductwork,
- SANS 1409: Outlet sockets and probes for medical (gas and vacuum) services used in hospitals,
- SANS 1409: Part 3 Handling and storage of Medical Gas,
- SANS 1424: Filters for use in air-conditioning and general ventilation,
- SANS 1453: Copper Tubes for Medical Gas and Vacuum systems,
- SANS 14644-1, Cleanrooms and associated controlled environments - Part 1: Classification of air cleanliness,
- SANS 14644-2, Cleanrooms and associated controlled environments - Part 2: Specifications for testing and monitoring to prove continued compliance with ISO 14644-1
- SANS 14644-4, Cleanrooms and associated controlled environments - Part 4: Design, construction and start-up,
- SANS 1474: 1988 Uninterruptible Power Supplies,
- SANS 7396-1: Medical gas pipeline systems Part 1: Pipeline systems for compressed medical gases and vacuum,
- SANS 7396-2: Medical gas pipeline systems Part 2: Part 2: Anaesthetic gas scavenging disposal systems,
- SANS 50081: Safety rules for the construction and installation of lifts — Particular applications for passenger and goods lifts,
- Any other applicable Laws or Regulations.
Chartered Institution of Building Services Engineers (CIBSE), 1999. Environmental design CIBSE Guide A. London: CIBSE.
Chartered Institution of Building Services Engineers (CIBSE), 2005. CIBSE Applications Manual AM10 Natural ventilation in non-domestic buildings. London: CIBSE.
Chartered Institution of Building Services Engineers (CIBSE), 2008. Lighting Guide 2: Hospitals and health care buildings. England: The Society of Light and Lighting.
American Society of Heating, Refrigerating and Air-Conditioning Engineers (ASHRAE), 2009. ANSI/ASHRAE/ASHE Standard 170-2008 Ventilation of
Health Care Facilities. Atlanta USA:ASHRAE.
American Society of Heating, Refrigerating and Air-Conditioning Engineers (ASHRAE), 2013. HVAC Design Manual for Hospitals and Clinics Second Edition. Atlanta USA:ASHRAE.
Further reading
- http://www.spaceforhealth.nhs.uk/ (National Health Service NHS website for UK guidance) website closed now
- http://healthfacilityguidelines.com/guidelines.htm (Health Facility Guides website for Australasian Health Facility guidance)
- CIBSE Guide A – Environmental Design
- CIBSE Applications Manual for Natural Ventilation – AM10
- CIBSE Applications Manual for Mixed Mode Ventilation. – AM13
- CIBSE Lighting Guide 2: Hospitals and Health Care buildings
- ASHRAE 170:2008
- HVAC Design manual for Hospitals and Clinics Second Edition – ASHRAE TC 9.6, 2013
- CIBSE Commissioning Code A
- BSRIA Application Guide 3/89.1
- ↑ de Dear, Richard; Brager, Gail (1998). "Developing an adaptive model of thermal comfort and preference". ASHRAE Transactions. 104 (1): 145–67.
LIST OF ABBREVIATIONS
A & E- Accident and Emergency Department
AHU- Air Handling Unit
CSSD- Central Sterile Supply Department
EMS- Emergency Medical Services
HCW- High Care Ward
HEPA- High Efficiency Particulate Air (filter)
ICU- Intensive Care Unit
NBR- National Building Regulations SABS 0400
NICU- Neonatal Intensive Care Unit
OT- Operating Theatre
SABS- South African Bureau of Standards
SANS- South African National Standards
SSO- Switched Socket Outlet
UDAF- Uni-Directional Air Flow
UPS- Uninterrupted Power Supply
URS- User Requirement Specification
LIST OF DEFINITIONS
For the purposes of these regulations, unless the context otherwise indicates-
“barrier isolator” refers to a device comprising an physical film separating an operator or clinician from a work process. The work process is maintained within an isolated environment which may be held at a positive or negative pressure.
"Central Sterile Supply Department (CSSD)" means a facility for the receiving, decontamination, preparation, packing, sterilizing, storing and issuing of sterile and disinfected instruments and other reusable materials. This facility is also known as the "sterilisation and disinfection unit"(SDU);
"cleaners' room" means a room for the storage of cleaning equipment, the drawing of clean water and the disposal of dirty water, washing and drying of cleaning equipment. This room may be combined with the dirty utility room;
"clean air" means air that does not contain a considered contaminant;
"clean utility room" means a room for the storage of sterilized packs, dressings-, sterile equipment and pharmaceutical supplies respectively; This area may also be used for a set-up area for ward procedures;
"considered contaminant" means any actual contaminant, surface or airborne, which may have a certain impact which for which measures are taken to avoid;
"cross contamination" refers to the contamination of any zone or surface by fomites, considered particulates aerosols, biological agents, fumes or gasses originating from another zone or surface.
"cross infection" refers to the spreading of an infection from one organism to another by cross contamination.
"department" means a grouping of accommodation which has a specific function within a hospital. Its area includes the associated internal or departmental circulation space
"dirty utility room" means a room used for collection and temporary storage of used equipment and general ward material; it can combine the activities of the sluice room, the soiled linen and waste room and the cleaners' room;
"emergency trolley/crash cart" means a mobile cart used for the storage of all appropriate resuscitation equipment and pharmaceuticals;
"equipment store" means a room used for the storing of monkey chains, traction kits and other general equipment;
"fresh air" means air drawn from outside air of a building and contamination sources;
"high care ward" refers to a ward for the care and management of specific types of patients requiring a minimum of eight hours nursing care per patient day;
"holding area" means an area or room where pre-operative patients in transit to a procedure room/theatre are identified and continuously monitored by nursing personnel;
"induction room" means an area where patients are prepared for surgery/invasive procedures prior to being transferred to the operating theatre;
"intensive care unit" means a unit designed, staffed and equipped for the care and management of specific patients, (e.g. medical, cardiac or post-operative) requiring a minimum of twelve hours nursing care per patient day or for the care of a patient who requires ventilation, continuous invasive monitoring, invasive care, or who is clinically unstable and whose life is at risk;
"main kitchen" means a facility suitably finished and equipped for the receipt, storage and preparation of meals, special diets and beverages;
"maternity unit" means a unit where antenatal care is provided, babies are delivered and postnatal care is given to mothers and infants;
" midwife obstetric unit (MOU)" means a maternity unit usually attached to a clinic or a community health centre (CHC), which is staffed by nursing sisters or midwives;
“milk kitchen” means an area for the preparation of feeds for babies which must be separate from the hospital kitchen or ward kitchen. It must contain a clinical wash hand basin;
"mortuary" means a facility that receives, holds and allows for the identification of bodies of patients who died in the wards, theatre or casualty department, or who were dead on arrival at the facility; a facility which complies with the
"neonatal unit" means a facility for premature and new born babies requiring incubation, specific care and monitoring;
"nurse station" means the control point for all activities in the patient care areas;
"nursing unit or ward" means a unit with the facilities to accommodate patients as specified in this regulation;
"operating room” means a room within an operating theatre suite in which surgical or other invasive procedures are carried out;
"operating suite" refers to rooms within the demarcated area where surgical interventions are performed or support is provided to these surgical activities;
"patient room" means a room where the patient can be accommodated;
"procedure room" means a room in which certain restricted procedures generally taking less than one hour can be performed without making use of general anaesthetic, e.g. endoscopies, procedures under local anaesthetic such as suturing of lacerations, removal of skin lesions, biopsies, closed reductions and other similar procedures; May be situated outside the operating suite;
"recovery room/ area" means the section of the operating suite specially set aside for the immediate post-operative recovery, resuscitation, nursing and special care of patients, until such time as such patients are considered to have recovered sufficiently to be safely removed from the operating suite;
"sluice room” means a room used for the emptying, cleaning and storage of bedpans and urine bottles; It can be combined with the activities of the soiled linen and cleaners' rooms in the dirty utility room;
“specialised area” means any clinical area rendering specialised services such as intensive care, high care, or rehabilitation, for which additional space around the patient is required;
"soiled linen and waste room" means a room used for the collection and temporary storage of soiled linen and waste; May be combined with the dirty utility room
"treatment room" means a room used for treatment of patients in the wards, containing a clinical wash hand basin;
“ventilation” means “The process of supplying air to or removing air from a space for the purpose of controlling air contaminant levels, humidity or temperature within the space”. ASHRAE Standard 62.1-2007, Section 3
“validation” means the method of proving and documenting that an installed system or process performs reliably as intended and required.
“natural ventilation” means “Ventilation provided by thermal, wind, or diffusion effects through doors windows or other intentional openings in the building." ASHRAE Standard 62.1-2007, Section 3
"ward kitchen” means the room that forms an integral part of a nursing unit or units, for the preparation of snacks and beverages; It also includes the area for the heating, storage and refrigeration of meals;
"uninterrupted power supply" means a battery system, which in the event of a normal mains supply failure will provide immediately the electrical supply for essential equipment and lighting.