Infection Prevention and Control/Air Disinfection: Difference between revisions
| Line 83: | Line 83: | ||
{| class="wikitable" | {| class="wikitable" | ||
!ZONE !!EYE LEVEL(m)!!ESTIMATED DAILY EXPOSURE TIME (h)!!IRRADIANCE LIMIT (uW/cm²) | !ZONE!!EYE LEVEL<br>(m)!!ESTIMATED DAILY <br>EXPOSURE TIME <br>(h)!!IRRADIANCE LIMIT <br>(uW/cm²) | ||
|- | |- | ||
|Corridors (no waiting)|| 1.7|| 1 ||1.6 | |Corridors (no waiting)||1.7||1 ||1.6 | ||
|- | |- | ||
|Indoor Waiting Areas|| 1.7|| 3 ||0.5 | |Indoor Waiting Areas|| 1.7||3 ||0.5 | ||
|- | |- | ||
|ICU ||1.7 <br> | |ICU ||1.7<br>1.0||4<br>6||0.4<br>0.3 | ||
1.0 | |||
|4 <br> | |||
6 | |||
|0.4<br> | |||
|- | |- | ||
|Bedrooms/Offices|| 1.7 2 0.8 | |Bedrooms/Offices|| 1.7<br>1.2|| 2<br>4|| 0.8<br>0.4 | ||
|- | |- | ||
|Wards/Dormitories|| 1.7 2 0.8 | |Wards/Dormitories|| 1.7<br>1.2|| 2<br>4|| 0.8<br>0.4 | ||
|- | |- | ||
|Isolation Rooms|| 1.7 2 0.8 | |Isolation Rooms|| 1.7<br>1.2|| 2<br>4|| 0.8<br>0.4 | ||
|- | |- | ||
|Consultation Rooms|| 1.7 2 0.8 | |Consultation Rooms|| 1.7 ||2 ||0.8 | ||
|- | |- | ||
|Bronchoscopy rooms|| 1.7 2 0.8 | |Bronchoscopy rooms|| 1.7 ||2 ||0.8 | ||
|- | |- | ||
|Autopsy Rooms|| 1.7 2 0.8 | |Autopsy Rooms|| 1.7 ||2 ||0.8 | ||
|- | |- | ||
|Dining Halls|| 1.7 2 0.8 | |Dining Halls|| 1.7 ||2 ||0.8 | ||
|- | |- | ||
|Dormitories|| 2.1 8 0.2 | |Dormitories|| 2.1 ||8 ||0.2 | ||
|- | |- | ||
|Other areas|| 1.7 4 0.4 | |Other areas|| 1.7 ||4 ||0.4 | ||
|- | |- | ||
|} | |} | ||
Revision as of 17:20, 14 May 2020
IMPLEMENTATION of UPPER ROOM UVGI - AN ABRIDGED GUIDE
1 Introduction and context This guide is a compilation of current best practice and knowledge regarding the design, development and operation of indoor room air ultraviolet germicidal irradiation (UVGI) systems for reducing the rate of transmission of airborne diseases such as tuberculosis (TB). This guidance document is not intended for the applications of water or surface disinfection. 2 Effectiveness of UV The disinfection effectivity of UVGI and the susceptibility of airborne microorganisms including M. tuberculosis (TB) bacilli (peak sensitivity at around 265nm) have been scientifically proven. 3 Safety UV-c has a lower skin penetration depth, thus does not easily cause skin irritation or cancers when compared to UV-a and UV-b found in sunlight. UV-c does cause eye irritation at high exposure levels.
Therefore, UVGI in occupied rooms should not exceed an exposure dose of 6 mJ/cm² (for mercury vapour lamps at 254 nm 15) and 3.8 mJ/cm² (at 265nm) per 8h. The potential of high UV intensities being reflected from certain materials (e.g. reflectors of regular open luminaires, windows, exposed ducting and metallic or high gloss architectural finishes) into the occupied portion of the room must be considered by designers and users. During any work in the upper room or with open UVGI devices, eye and skin protection should be worn. 4 Applications and definitions The application of UVGI should not be seen as a substitute for, rather a component of, a comprehensive IPC policy. 4.1 Upper room UVGI These devices irradiate air and inactivate pathogens in the unoccupied zone of the upper room. During normal air circulation the air exchange between the upper and lower room, reduces the concentration of viable airborne pathogens in the whole room. 4.2 Air cleaners Room air cleaners consisting of enclosed UVGI lamps in housings, circulate air to achieve a measure of clean air delivery to the room. The advantages relate to safety aspects and lower maintenance costs. 4.3 Whole room UVGI Whole room UVGI disinfection attempts to sterilise room surfaces by exposing the entire room volume to high UV fluence levels. This method is ineffective for reducing airborne transmission within occupied rooms. 5 Planning and procurement The uncontrolled use of UVGI without a comprehensive management policy can result in fruitless expenditure and create a false sense of protection, thereby potentially exposing building occupants to increased risk. The management policy should align with the regional and national IPC policies and should include sourcing and procurement, maintenance, training, decommissioning and disposal of UVGI devices. 6 Design and installation 6.1 Design lifecycle The UVGI design life cycle should include system planning, space evaluation, design and review, control of design and commissioning documents. Risk prioritization and reverting to more conventional infection control strategies should be considered. Interference from obstructions (light fixtures, beams, ductwork, piping, furniture, equipment or non-prescribed lighting diffusers) to airflow around the device or radiation from the device must be taken into account. Two design processes are briefly described. 6.2 Radiometric design process Two rational design methods are available. The first process follows the guidance of CIE 155:200315 and the design completion will need quantification and verification *of: • Selected UVGI device’s radiometric data • Considered pathogen’s UV-c sensitivity (Z-value) • Considered pathogen’s infectious dose • Air exchange rates and ventilation efficiency parameters The design objective is a prescribed reduction in transmission of 80% or higher.
The second rational design process is available through the application of design software targeting prescribed whole-room radiant flux levels. However, this method is only appropriate for open type UVGI devices. Prescriptive design process The prescriptive design process aims to determine the required number of UVGI devices for the considered indoor space and the resultant occupancy limits in accordance with the WHO recommended minimum ventilation rate of 80 l/s per person. This design process is applicable for well-mixed room air, achieved through the installation of mixing fans. If the considered room has an existing and functional mechanical ventilation system, the outside air portion of that system’s ventilation rate should be included in the calculation to increase the occupancy limit determination. The design process assumes that UV-reflective surfaces do not affect eye safety.
The following set of device and application data is required: Total maintained UVGI radiant flux (ФT, mW) of selected devices, measured in accordance with SATS 1706:2016 as amended and the UVGI measurement and instrumentation and calibration plan The selected device’s minimum equivalent clean air delivery rate (CADRe, l/s). Room volume (Vr, m³), occupancy profile (Np, number of persons) and effective mechanical ventilation rate (Qv, l/s) of considered room.
〖Number of devices〗_Ф=(14×V_r)/Ф_T CADR_e=((80×N_p )-Q_v )/〖Number of devices〗_CADR
It is strongly recommended that the room be labelled with clear and informative signage to indicate the TB related occupancy limits with the UV system turned on and turned off. Acceptance criteria Design requirements for effectiveness can be assessed through compliance with the following: Minimum required UV volumetric fluence rate (MRU) of 14mW/m³ or A UVGI equivalent ventilation rate of the product of 80 l/s per person and the typical peak number of room occupants. This CADRe rate determines the total required UV-c output. Any shortfall between the required and the actual ventilation rate or MRU should be corrected for increasing by the number of UVGI devices installed, as determined using the formulae in section 6.3. The number of devices required should not be less than the minimum number determined, or exceed this value by more than 1. Alternatively, for rooms with unknown occupancy levels, the CADRe resulting from the number of devices determined by the prescribed MRU could be used to define the safe occupancy limit for that room The UV dose should inactivate airborne infectious agents by at least 90% (D90) or a percentage equivalent of the ventilation rate of 80l/s/per person as recommended by the WHO.
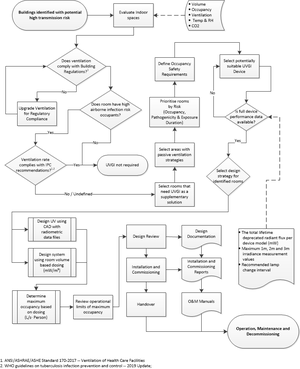
1.1 Lamp ageing A 100-hour lamp burn-in is recommended prior to the device characterisation or system verification tests. The lamps of dimmable systems should be burnt-in at full output for the initial seasoning period.
1.1 Factors affecting performance UVGI disinfection performance is dependent on good air movement rates either through natural convection or mechanically aided (e.g. paddle fan). The effectiveness may be reduced if the mechanical ventilation rates in a room are increased together with equivalent room air mixing. However, as ventilation is a primary environmental control against indoor airborne transmission, its maximum capacity within the considered area should be determined and targeted before implementing a UVGI solution.
1.1 Installation The device must not be able to tilt or swing under normal operation. The minimum installation height of the lower horizontal plane of any open UVGI device above the finished floor level is determined from the table below.
| Ceiling Height (m) | Recommended Mounting Height (m): | ||
|---|---|---|---|
| Corner type (90°) | Wall type (180°) | Pendant type (360°) | |
| 2.4 | 2.2 | 2.2 | 2.4 |
| 2.7 | 2.3 | 2.3 | 2.4 |
| 3.0 | 2.4 | 2.4 | 2.4 |
In the typical application of 254nm devices, occupant exposure shall not exceed 0.40W/cm2 for a period of time equivalent to 4 hours per day. The strategy for determining equivalent acceptable exposure levels in the lower room in typical healthcare settings is represented in the table below.
| ZONE | EYE LEVEL (m) |
ESTIMATED DAILY EXPOSURE TIME (h) |
IRRADIANCE LIMIT (uW/cm²) |
|---|---|---|---|
| Corridors (no waiting) | 1.7 | 1 | 1.6 |
| Indoor Waiting Areas | 1.7 | 3 | 0.5 |
| ICU | 1.7 1.0 |
4 6 |
0.4 0.3 |
| Bedrooms/Offices | 1.7 1.2 |
2 4 |
0.8 0.4 |
| Wards/Dormitories | 1.7 1.2 |
2 4 |
0.8 0.4 |
| Isolation Rooms | 1.7 1.2 |
2 4 |
0.8 0.4 |
| Consultation Rooms | 1.7 | 2 | 0.8 |
| Bronchoscopy rooms | 1.7 | 2 | 0.8 |
| Autopsy Rooms | 1.7 | 2 | 0.8 |
| Dining Halls | 1.7 | 2 | 0.8 |
| Dormitories | 2.1 | 8 | 0.2 |
| Other areas | 1.7 | 4 | 0.4 |