Clinical diagnostic laboratories
Please help to expand this page. |
Planning and Design
This section provides design guidance for laboratory location, location of staff and pre-analytical areas, sizing and location of laboratory furniture, sizing and location of storage areas, and designing current laboratories for future needs.
The laboratory design objective is primarily to develop environments in which laboratory activities can be conducted without compromise to laboratory processes, patient dignity and occupational health and safety.
Spatial Planning
This guidance document is written within an environment of rapidly developing and expanding point-of-care diagnostic techniques and technologies. In this environment much of the laboratory procedures is moving out of the laboratory and to the patient’s bedside. The impact on the laboratory is an emerging requirement for additional storage space and for more open and flexible laboratories. For this reason the development and planning of any laboratory cannot be undertaken without an informed understanding of the techniques and technologies that will be employed in that laboratory. Where this guidance document refers to separate clinical activities, it should be assumed that these functions, unless otherwise specified or due to or technical restrictions, may be conducted within one open plan area at the discretion of the service provider.
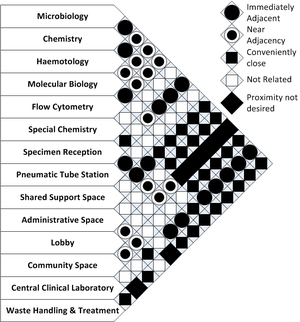
The questions to be asked and answered during the space planning stage of design relate to the adjacency of the required spaces and their inter-relationships.
An overall adjacency matrix is presented to facilitate this planning of these spaces. This matrix should be read in the context of the services that will be provided by the laboratory being planned. It will be seldom that all the spaces or function specified will be required in a single facility.
The space adjacency matrix presented is adapted from Guidelines for Laboratory Design: Health, Safety, and Environmental Considerations [1]
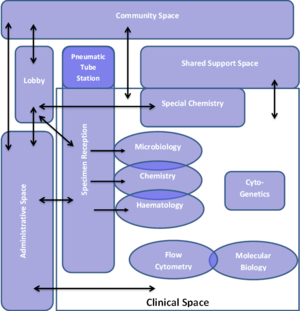
From the adjacency matrix, a space planning inter-relational diagram such as one depicted in the Lab Interrelationship Diagram. is developed. This diagram subsequently informs the development of the facility layouts. The example given indicated the flow of samples in the facility. Similarly the flow of staff and patients should also be described on such a diagram. This planning step is useful to ensure the relationships between processes and spaces are well understood and accommodated in this highly technical and variable environment. Waste storage and treatment areas are not shown in the example given.
The basic laboratory facility within a hospital or healthcare facility may accommodate one or more of the following clinical diagnostic activities:
- Clinical Pathology, which may include:
- Hematology
- Chemistry
- Microbiology
- Immunology
- Anatomical pathology, which may include:
- Histology
- Cytology
When planning the location of a laboratory within the hospital, the following factors should be considered and addressed:
- The location should not be utilised as an escape route by patients or staff from other hospital department wards.
- Laboratories should be located such that they cannot be used as thoroughfares for hospital staff or the general public.
- Laboratories are considered to be sources of infection and fire hazard areas and therefore should not be located in areas frequented by the public.
- Laboratories should not form part of general emergency egress routes. However, emergency egress routes should be planned from laboratories without compromising contaminations control and security requirements.
- Facilities should be designed to comply with the National Building Regulations as amended.
Facility sizing
The following matrix offers guideline space planning for concept development benchmarking and high level costing of laboratory facilities.
Please help to expand this page. |
| SPACE PER HOSPITAL (m²) | |||
| Laboratory Areas | 0-200 Beds | 200-400 Beds | 400-800 Beds |
| Community | |||
| Staff | |||
| Administrative | |||
| Pre-analytical | |||
| Analytical | |||
| Storage | |||
| Waste and Technical | |||
Functional areas
The functional areas in a laboratory can be described as, community areas, staff areas, pre-analytical areas, analytical areas and waste storage and treatment areas.
Staff Areas
- Staff facilities should include:
- Protective clothing storage. This is normally in the form of individual lockers. Individual lockers give staff place to store street garments and valuables as required.
- A tea room for resting, socialising and storage and preparation of beverages.
- Education and training facilities – These are sometimes provided at central or tertiary facilities.
- Where full garment changing is required separate changing facilities should be provide for both male and female. Where lab practice deems it acceptable to wear lab coats over street clothes, dedicated change rooms may not be required.
- The staff compliment size determines the number of changing spaces and lockers. This consideration should include capacity for researchers and trainees where required by the service level agreement.
- Separate male, female and accessible toilets should be provided.
Pre-analytical areas
- Pre analytical areas include spaces for administrative and logistic operations. This includes spaces for sample receipt, storage and dispatch.
- Administrative offices should are used multiple people simultaneously for desk-based work. Administrative workspaces should be large enough to accommodate daily filing, stationary and a workstation.
- Each laboratory facility should have only one specimen reception area. This area should receive samples, capture and process test request and distribute the samples to the appropriate laboratory.
- Specimen sorting may be required. For this operation, a specimen sorting area is required immediately adjacent to the specimen receipt area, prior to dispatch to the relevant laboratory.
- The sorting area requires a number of individual work-stations for processing and packing of discrete sample batches to prevent mix-ups.
- Each work area should have adequate lighting and a space for a PC workstation to complete any electronic record workflow tasks.
- Hand wash facilities are required in all areas where specimen handling occurs.
- A room should be provided for the collection of primary samples. This area should be designed considering patient comfort, privacy (including acoustic privacy) and dignity without compromising the requirements of efficient sample collection. These areas should also be designed to accommodate the accessibility needs of persons with disabilities.
Analytical areas
General Requirements
- The analytical areas for functional clinical laboratories include chemistry, haematology, pathology and cytology laboratories. This document is limited to the requirements of laboratories classified up to biosafety level 2. The range of services offered in a clinical laboratory varies from facility of facility and is dependent of the local service level agreement in place.
- The laboratory design objective is primarily to develop environments in which laboratory activities can be conducted without compromise to laboratory processes, quality and occupational health and safety. Sources of risk and recognised harm should be identified and mitigated against.
- A consultative workflow analysis is required at early conceptual design stage in order to resolve the following:
- Identification of incompatible processes or activities which need to be separated spatially and physically such that they require individual areas. Cross contamination between processes should be prevented.
- Modern clinical laboratory workflow and equipment does not require physical separation of activities into separate areas and an open-plan laboratory is acceptable except for the following:
- TB culture and diagnostics
- Microbiological Laboratory
- Cell culture laboratory
- Each laboratory module must be equipped with a wash-hand basin. See further for details.
- Sufficient space should be provided for automatic lab equipment. This equipment often requires access right around and has a continuous linear arrangement. Power, water and gas services to this equipment should be provided from overhead installations.
- Laboratory isles should not be narrower than 700mm with main isles no narrower than 1050mm. Main aisles used for emergency egress must comply with requirements of the National Building Regulations. Access for equipment movement and replacement should also be considered. Emergency exit routes should be clear of furniture and equipment.
- Door openings should not be less than 900mm. Refer below for further details.
- Biosafety cabinets should not be placed along internal staff traffic routes and should not have an isle with staff passing in front of the cabinet. These cabinets should not be placed near operational doors or general ventilation terminals
Cytopathology and Histopathology Laboratories:
- Storage capacity for cytopathology and histopathology slides, blocks and records is the major consideration. Storage capacity needs to be determined from the annual case-load.
- Consideration should be given to the adopting off-site storage. Off-site storage conditions and distances need to be assessed.
- Building structural capacity and floor loading should be considered for storage areas.
- Storage space for histopathology slides and blocks should be provided, based on annual workload.
Microbiology Laboratories:
- Centrifuges generate significant noise and vibration. Large centrifuges can also consume considerable power and generate heat. These effects should be isolated so as not to affect the rest of the equipment or activities in the lab such as balances and microscopy.
- Balance tables should be provided for scales and balances.
- Microscopy work requires facilities for staining of slides and imaging. Dedicated benches which are free of rotating or vibrating equipment should be provided for imaging processes.
- Cell and Tissue Cultivation Laboratories:
- Dedicated cell culture laboratories may be required for genetics or virology testing.
- Cell culture and labs require 10-20 air changes per hour of EN779-F9 filtered air. Open samples should be manipulated in biosafety cabinets.
- In-vitro fertilisation (IVF) labs should be finished in non-VOC emitting materials and should be ventilated with activated carbon filtration. Ventilation equipment and components should additionally be non-VOC emitting.
- A cell culture laboratory for the cultivation of cells and tissues may be required if genetics and virology testing is planned. Class IIA1 biosafety cabinets in accordance with the WHO Laboratory biosafety manual, Third edition (2004) will be required for manipulation of samples. The compulsory specification VC8041 published by government notice R93 (2001) requires a down-flow velocity of 0.5m/s for these cabinets. This velocity is, however, likely to affect scales operating within the cabinet. A selection of cabinets with a more moderate velocity in the range of 0.25 m/s is recommended as per the SANS 12469:2002.
- Cell and tissue cultivation laboratories should accommodate at least:
- one bench-mounted microscope
- an under-bench fridge and freezer
- a bench-mounted centrifuge
- a floor-standing incubator
- a sink with associated services
- separate hand wash basin with associated services
Point-of-care testing (POCT):
- Typical point of care testing equipment includes:
- Reagent-impregnated strips
- Single test equipment for glucose or cholesterol and
- Fully automated instruments, such as blood glucose monitoring systems and blood gas/critical care analyser.
- Where point of care consulting areas are provided in a primary care areas these should be equipped with an examination couch, desk and chairs, wash-hand basins and point of care testing equipment.
- A point of care laboratory module should be 3m X 3m and allow for back-to-back working at laboratory benches as a minimum.
- A point of care laboratory module should include:
- a sink with associated services
- laboratory benching for testing equipment and computers
- floor space for under-bench refrigerators,
- separate wash-hand basin facilities
- storage space should for equipment and supplies
Storage
This section details location and spatial planning of storage areas for specimens, chemical reagents and other laboratory consumables, and assists in determining storage requirements per functional laboratory.
- Relevant storage space and conditions should be provided to ensure the continuing integrity of samples, slides, histology blocks, retained micro-organisms, documents files, manuals, equipment, reagents, laboratory supplies, records and results[2].
- Supplies and service equipment should be stored in non-laboratory support spaces
- Waste treatment and storage may be done at the laboratory or by non-laboratory support services.
- Separate storage facilities should be provided for the following:
- blood and blood products
- chemicals and reagents
- clinical material
- drugs, vaccines and other therapeutics
- hazardous substances
- process records
- quality records
- Storage and disposal of dangerous materials should be those specified by relevant standards and regulations (SANS 15189:2009).
- Sufficient space should be provided so that incompatible waste and non-waste chemicals and gases can be physically separated and stored.
- Compatible non-flammable laboratory chemicals could be stored together. These should be stored in a poisons cabinet where appropriate.
- A flammable material store can be provided for day-today storage of flammable chemicals.
- Bulk storage of flammable materials should be stored in a secure ventilated, flammable goods store that is external to, but easily accessible from the laboratories.
- Extremely and highly flammable liquids should be stored in accordance with Government Notice R1031 (1986)
- Refrigerators used for storage of flammable volatile solvents should have intrinsically safe or non-incendive motors, lighting and switchgear.
- Stores that are easily accessible from the relevant laboratory areas are required for working stocks of materials and equipment.
- The chemical store should have a preparation area for weighing and preparing reagents for use in the laboratory areas.
- Provision should be made for the storage of glassware and other equipment.
- Ergonomic and safety considerations should be made when designing laboratory shelving. A recommendation is given as follows[3]:
- Shelving should not be installed which require laboratory staff to reach 30 cm above shoulder height, and extend arms greater than 30 cm while holding objects 16 kg or less when standing on the floor or on a 300mm stool
- Oxidising agents should not be stored on wooden shelves.
- A storage area for pressure vessels, compressed gas cylinders and pressurised manifolds where:
- Gasses should be segregated by hazard class and flammable gases should not be stored with oxidizing agents.
- They are protected from external heat sources, radiant heat, electric arcs or high temperatures.
- They are in a well-protected, well ventilated, dry location, at least 6m from highly combustible materials
- Refer to the Pressure Equipment Regulation of 2009 for further requirements.
Walls, doors and architectural details
This offers guidance on the planning, location and detailing of architectural details and furniture. This section should be read in conjunction with the IUSS Materials and finishes guidance document.
- The laboratory should be entirely enclosed within the walls floors and ceilings of a building. No functional clinical portion of the laboratory should include outside areas.
- Openable windows in clinical laboratories are discouraged. The WHO does however advocate naturally ventilated TB diagnostic laboratories and, should this concept be adopted, it should be a free-standing unit, physically isolated from the rest of the laboratory facility. Any openable windows should include security bars which do not impede the mechanical operation of the window and should also include insect and vermin screens.
- Laboratory doors should be fitted with automatic door closers and viewing panes. Emergency door should swing in the direction of egress and doors between rooms with a cascading pressure differential should swing in the direction of the higher pressure.
Open vs Closed Laboratories
This section addressed the requirement for modern modular laboratories to be designed around a concept of long term flexibility and the accommodation of unknown future laboratory trends and technologies.
- The long term strategy of any a laboratory within the healthcare service and the rapidly changing nature of laboratory technologies and practices should inform any planning of new or revamped facilities. While new technologies may bring the advantage of ever diminishing equipment sizes, the emergence of new equipment for previously manual or non-existent techniques has the counter effect of increasing the space and storage requirements for laboratories.
- With the trend towards automation of laboratory services new fully automated equipment can be large heavy and bulky. Present and future consideration should be given to providing contingency access for the installation and removal of such equipment. Provision of access and space should also be made for the inclusion of related support services.
- The provision of a generic open laboratory space can accommodate a range of pathology functions. This flexibility is maintained, even in an essentially unchanging or standardised repetitive framework of services, by relocating people and services within the space to accommodate new needs and equipment.
- A generalised lab arrangement should be developed during the design stage. This arrangement should be able to host the widest foreseeable range of services that may need to be presented at that laboratory.
- The design stage assessment of the physical aspects and requirements should include:
- Availability of space for alterations and additions, including office space and staff facilities.
- Type of construction and compliance with national building regulations.
- For retrofitting projects, the condition and age of the building and related engineering services.
- Fire precaution requirements
- Physical constraints such as load bearing walls and load capacity of floor slabs.
- Energy usage and available capacity.
- Decommissioning and disposal requirements. When selecting equipment, account should be taken of the use of energy and future disposal (care of the environment) (SABS, 2009).
- Modular furniture, adjustable height tables and movable furniture with standardised dimensions are beneficial as these enable the reconfiguring or removal of workstations as technology, processes and services change [1].
- The design stage assessment of the physical aspects and requirements should include:
Laboratory Equipment
This section addresses building infrastructure to accommodate laboratory equipment, including Laboratory Hoods and Fume Cupboards, Biosafety Cabinets, Fridges and Centrifuges.
Laboratory Hoods, Fume Cupboards and Biosafety Cabinets
- Valves, electrical outlets and utility switches serving hoods should be placed at readily accessible locations and should be clearly marked.
- Planning of location and routes for the extraction ducting and services to the external extraction fan should be considered by the architectural team even if while the fume cabinet may be supplied by the laboratory service provider.
- Manifolding of fume hood exhaust ducting from adjacent laboratories should be avoided.
- Fume hoods should not be the sole means of laboratory room ventilation. The quantity of air extracted by fume hoods should be replenished with tempered outside air by a dedicated and interlocked mechanical ventilation system.
- Room air currents near the fume hood should not exceed 0.1m/s to ensure hood containment.
- Fume cupboards should not be positioned such that lab traffic and escape routes would not necessitate passing directly in front of the cupboard.
- The design, installation and operation of biosafety cabinets should comply with requirements of the following:
- SANS 10226:2009 The installation, post-installation tests and maintenance of microbiological safety cabinets
- SANS 12469:2002 Biotechnology - Performance criteria for microbiological safety cabinets
- VC8041 2001 The compulsory Specification for Microbiological Safety Cabinets
Refrigerators and freezers
- Refrigerators and freezers should not be located in one room unless the air-conditioning system can accommodate the high heat gain of this equipment.
- Where specimen and reagent storage capacity demands the use of cold rooms rather than refrigerators, these rooms should be maintained at +4˚C. These fridges should include insulated floor drainage and modular shelving.
Engineering Services
- Flexibility in the provision of support services such as electricity, water, vacuum and gasses can be achieved by the adoption of an open ceiling grid where neatly installed services can be easily identified, maintained and reconfigured as required. These services should be laid out on a regular grid with provision for regular connection points.
- Where services cannot be brought down through ceiling mounted pendants, floor mounted service islands or spines should be created to which mobile furniture and equipment can be attached.
Waste Treatment and Disposal
- The management treatment and disposal of healthcare waste must be in accordance with the requirements of the SANS 10248 Management of healthcare waste, set of standards (SABS 2004). This set of standards includes:
- SANS 10248 Management of healthcare waste[4]. This document specifies criteria for the segregation, collection, movement, storage and disposal of waste materials within health care.
- SANS 10248-1 Management of healthcare waste - Part 1: Management of healthcare risk waste from a healthcare facility.[5] This document lays down minimum provisions for the safe and effective management of healthcare risk waste generated by healthcare facilities and other places where healthcare professionals work in order to reduce potential risks to humans and to the environment. The management of healthcare risk waste covers the generation, the packaging, the treatment and the disposal of waste.
- SANS 10248-2 Management of healthcare waste - Part 2: Management of healthcare risk waste for healthcare facilities and healthcare providers in rural and remote settings.
- SANS 10248-3 Management of healthcare waste Part 3: Management of healthcare risk waste from minor generators - Registered healthcare professionals and non-healthcare professionals
- Additional legislation and regulations that should be considered wen planning for the generation, treatment, disposal and transport of waste or materials that contain or potentially contains human pathogens
- All waste generated in the laboratory should be considered for safe disposal. Laboratory waste
- should be disposed of in observation of the following South African legislation:
- Hazardous Substances Act (Act 5 of 1973)
- Environment Conservation Act (Act 73 of 1989)
- Occupational Health and Safety Act (Act 85 of 1993)
- National Water Act (Act 36 of 1998)
- The National Environmental Management Act (Act 107 of 1998)
- Municipal Structures Act (Act 117 of 1998)
- Municipal Systems Act (Act 32 of 2000)
- Mineral and Petroleum Resources Development Act (Act 28 of 2002)
- Air Quality Act (Act 39 of 2004)
- National Environmental Management: Waste Act, 2008 (Act 59 of 2008)
- National Road Traffic Act, 1996 (Act 93 of 1996)
- The following considerations should be made when planning for laboratory waste discharge to the domestic sewer system:
- Even though most sewers are can accept a tolerable level of infectious material, the healthcare facility has a duty of care not to discharge healthcare waste other than domestic sewage to a domestic sewer. Where needs exist to discharge waste to the domestic sewer, provision shall be made to adequately treat the waste to make it safe for discharge.
- The following wastes shall not be discharged to the domestic sewer:
- listed chemicals such as mercury
- pharmaceutical waste
- Alternatives to direct disposal to domestic sewer, which can be planned for, include destruction and disinfection methods such as:
- Incineration
- Autoclaving
- Microwaving
- Chemical disinfection
- The size of the waste treatment and storage areas should be determined by the frequency of the collection and the amount of waste generated. This would be directly affected by the proximity of the facility to waste management service providers.
- Sharps should be discarded at point of use and sharps bins should be provided for this purpose.
- Biosafety level labs should have direct access to autoclaving facilities. These autoclaves should preferably be dedicated to the lab for waste management purposes.
- Chemistry labs shall implement chemical dilution traps within all sinks and hand wash basins. Transparent traps are preferable.
- Metal pipework is not suitable for use in pathology laboratories as these are subject to corrosion by discharged chemicals and biological waste. Non corruptible materials such as glass and plastics may perform better assuming the selection of a suitable solution gives consideration to the temperature of the discharged fluids, the corrosive and volatile nature of the discharge and the mechanical limitations of the material selected.
- Glass, polypropylene, and other plastics are suitable, but consideration should be given to the chemical characteristics, temperature of the fluids discharged, and arrangements for fixing and supporting the drain[6].
- The designer should liaise with the local authority to establish the maximum permissible discharge quantities of laboratory waste.
General Utilities
This section discusses lab specific guidance relating to general engineering services and utilities. These utilities refer to electrical, plumbing and communications services. Design guidance of the building infrastructure for the location of general services. Design guidance could be specific to a functional laboratory and may infer from operational practices of the functional laboratory. For more specific guidance refer to the Building Engineering Services guidance.
Lighting:
- Laboratories should have adequate natural or artificial illumination to ensure visibility for diagnostic accuracy and operational safety. Uniformity of lighting and prevention of glare are as important at illumination levels.
Electrical:
- Worktop, flush mounted electrical outlets are not recommended as these areas have a high potential for liquid spillage.
- Sufficient power outlets should be provided for the equipment planned including an additional contingency quantity.
Plumbing:
- Drainage lines in the ceiling space of clean labs should be avoided. This pertains specifically to inspection and cleaning eyes.
- Hot and cold water systems:
- The floor below the emergency shower should be graded and drain into a suitable drainage point to prevent this water from running across the lab floor.
- The following guidance for drainage and waste systems is to be read in conjunction with Waste Treatment and Disposal above:
- Sink traps and piping to floor drops should be made of acid-resistant materials. Below ground, acid-resistant pipes should not be damaged by minor quantities of acids and solvents.
- Laboratory drain vents should be routed through the roof to outside and may not be manifold connected to sanitary vent piping
- Drainage lines from pathology laboratories should not be routed through other hospital accommodation such as critical care areas, operating theatres and catering departments as these drains can harbour high risk pathogens
- Autoclaves, with the exception of bio-secure autoclaves, should not be connected directly to the sewer drainage system but should be connected with an air gap using a tundish to prevent back-contamination.
- Waste lines from autoclaves should be designed with the temperature of the liquid discharge in mind. Where boiling water is discharged into sewer drainage or external gulleys, provision should be made for the management of any steam. High quantities of steam can travel back up the drainage system into adjacent areas.
- Floor gullies should be avoided.
Ventilation
- The reader is advised to read this section in the context of the Building Engineering Services guidance as many of the principles of airborne contamination control are introduced and developed therein.
- Where general laboratories require artificial ventilation they should have a minimum ventilation flow rate of 6 ACH. BSL2 labs and higher shall have a minimum ventilation rate of 12ACH (SANS 10400-O).
- The use of fan-coil or split air-conditioning units to reject internal heat gains is acceptable in general labs but not on cell culture or IVF labs. These labs would need a dedicated ventilations system providing fine filtered air and ventilation rates as described above.
- Laboratories must be maintained at a pressure negative relative pressure to the corridor and less hazardous adjacent areas.
- Air exhausted from laboratory work areas should be vented directly to outside and not into plant rooms, interstitial spaces, service shafts and ceiling voids.
- Where add air fume hoods are considered for their potential energy savings, these units should be specified and validated such that the add air system does not cause turbulence and flow reversal at the sash hood. Add air systems should be considered as potentially risky. Add air systems may also create a level of operator discomfort during seasonal extremes of climate.
- Side wall grilles and diffusers should not be used as these will disturb the cabinet performance.
- Doors should be fitted with door closers including a damped slow latching feature. Fast swinging doors can create a piston effect in rooms and disturb cabinet airflows and performance.
- No laboratory ventilation ductwork may be internally insulated by any means.
- Exhaust stacks from fume hoods or exhausting biosafety cabinets should extend and discharge vertically upwards at a height of 3m above the roof of the building without any risk or re-entrainment of that air directly into any ventilation system or openable window. Laboratory exhaust shall discharge at or below head height in any trafficable or technical areas. Discharge velocities of 15m/s are recommended.
- Where the building profile does not permit high level discharge, dilution systems with very high discharge velocities could be considered under careful design and a strict validation plan. The risks of such an approach should be well understood and managed.
- Laboratory exhaust system fans should be located as near to the point of discharge as possible to ensure as much of the exhaust duct is under negative pressure as possible. All exhaust ductwork inside the building should be under negative pressure. Anti-backflow devices should be installed and discharges should be protected from high winds and adverse weather.
- Exhaust fans should be easily accessible for maintenance and should be clearly identified with durable weather proof labels indicating which laboratory and hood they are serving.
- A separation distance of 25m is recommended between laboratory exhausts and any ventilation opening.
- Staining areas should have bench extract systems that ensure air flows away from operator’s faces. Low-level extract should be provided adjacent to equipment for use when solvents are changed or when specimens in formaldehyde are opened[6].
Security and Access Control
- Laboratory infrastructure design planning should include the location and planning of security control and access points. Design guidance could be specific to a functional laboratory and may infer from operational practices of the functional laboratory.
- Access to, and use of areas affecting the quality of examinations should be controlled. Appropriate measures should be taken to safeguard samples and resources from unauthorised access[7].
- Access should be restricted to authorised personnel only. This could be attained through security measures, such as swipe cards, or locks to controlled entry points.
- Use of biometric fingerprint scanning systems can fail in settings where the occupants are required to wear surgical gloves or regularly work with harsh chemicals. Alternative scanning systems should be considered.
Materials
- The reader is advised to read this section in the context of the IUSS Materials and Finishes guidance document as many of the principles of airborne contamination control are introduced and developed therein.
- Surface materials should be resilient to scratches and chemicals such as acids, alkalis, detergents, disinfectants, solvents and staining fluids.
- Cellulose based substrates for laminated surfaces are not permissible in cell culture and IVR labs. Cellulose based surfaces are not permissible in pathology laboratories.
- Bench tops are to incorporate a faint lip to prevent spillage from seeping onto the floor.
- Tiled walls and floors are not permissible.
- Fiberglass building surface finishes are not permissible.
- Laboratory facilities should be designed to facilitate as well as withstand easy, frequent and thorough cleaning. Surface finished should therefore be impervious, resilient and continuous.
- Continuous coved cornices are at wall and floor interfaces. Coving radiuses of 40mm are recommended. Larger radiuses could prevent furniture legs from fitting closely enough to the walls. The interface between permanent immovable furniture and the floor should similarly be coved.
- Walls should be coated with a hard non-porous paint finish.
Special Laboratories
This section only refers to biological laboratories. It excludes forensic, nuclear and radiological laboratories.
Microbiological laboratories
This article describes limited building infrastructure guidance related to biological laboratories, up to and including Biosafety Level 4.
This document should be read in conjunction with the infrastructural requirements for biological laboratories as set out in Regulation 178 of the National Health Act of 2003 (added 2 March 2012)[8].
For microbiology, separate bio-safety level 3 laboratories (BSL3) are required if work is to be carried out on hazard group 3 organisms and specimens (R178[8]).
The following table gives guidance on the general requirements for biosafety laboratories.
Where no specific guidance is given for a specific laboratory level, the related recommendation described for lower level laboratories is implied.
| ● Mandatory
■ Mandatory (Animal bio-safety) ○ Recommended □ Recommended (Animal bio-safety) Ө Prohibited | ||||||
| REQUIREMENTS | Containment Level 2 | BIO-CONTAINMENT LEVEL: 3 AND ABSL 3 | BIO-CONTAINMENT LEVEL 4, ABSL 4 and BSL3 Ag Animal Rooms | |||
| ORGANISMS ARE NOT TRANSMITTED BY AIRBORNE ROUTE | ORGANISMS ARE MAINLY TRANSMITTED BY AIRBORNE ROUTE – (ADDITIONAL REQUIREMENTS) | CABINET CONTAINMENT | SUIT CONTAINMENT | |||
| A. | BIOSAFETY PRINCIPLES | |||||
| 1) | Hazardous biological agents, contaminated materials, waste and gasses shall be handled so as to prevent release. | ○□
Minimise release |
●■ | ●■ | ●■ | ●■ |
| 2) | Access shall be limited to authorized personnel only and communicated with signage | ●■ | ●■ | ●■ | ●■ | ●■ |
| 3) | Personal protective clothing and equipment required | ●■ | ●■ | ●■ | ●■ | ●■ |
| 4) | Personnel decontamination and washing facilities | ●■ | ●■ | ●■ | ●■ | ●■ |
| 5) | Facility shall be designed for surface decontamination. | ○□ | ●■ | ●■ | ●■ | ●■ |
| 6) | Dedicated process and laboratory equipment required | ○□ | ●■ | ●■ | ●■ | |
| B. | LOCATION AND ACCESS: | |||||
| 1) | Separated from public areas | ○□ | ●■
Note: Physical separation |
●■
Note: BSL 4 cabinet & suit labs in separate building or isolated zone |
●■
Note: BSL 4 cabinet & suit labs separate building or isolated zone | |
| 2) | Industrial processes should contain bulk viable organisms in closed systems which provide physical separation to the environment. | ●■ | ●■ | ●■ | ●■ | |
| 3) | Doors to containment areas lockable with access limited to fewest number of possible authorized personnel | ●■ | ●■
Note: By secure, locked doors Note: Only staff designated by the director of the establishment should have authority to enter |
●■
Note: By secure, locked doors Note: Only staff designated by the director of the establishment should have authority to enter | ||
| 4) | Doors leading between change rooms, showers and the containment areas shall be self-closing and interlocked to prevent inter-leading doors from being opened simultaneously. | ○□
Note: (interlock, visual or audible alarm, or protocols are accepted means |
●■
Note: (interlock, visual and audible alarm are accepted means) |
●■
Note: (hardware interlock is the only accepted means) |
●■
Note: (hardware interlock is the only accepted means) | |
| 5) | Doors provide restricted access by installation of a controlled access system or equivalent. Access to be recorded / documented | ●■ | ●■ | |||
| 6) | Any electronic locking systems are backed up by physical key-lock systems and an alarmed emergency exit override. | ●■ | ●■ | ●■ | ||
| 7) | Two-person rule is implemented whereby no individual ever works alone. | ○■
Note: Based on site-specific risk assessment |
| |||
| 8) | Containment room doors have appropriate signage | ●■ | ●■ | ●■ | ||
| 9) | Office areas are located outside containment areas.
Paperwork stations can be within containment space provided they are located away from containment work areas. |
●■ | ●■ | ●■ | ||
| 10) | Containment areas must be separated from areas within building having unrestricted traffic flow by an anteroom and changing facilities. | ●■ | ●■ | ●■ | ||
| 11) | Any combination of rooms through which the containment area is entered and which performs a zone separation function shall include facilities for separating clean and dirty clothes. | ●■ | ●■ | ●■ | ||
| 12) | Exit from containment area provided with a walk-through compulsory water shower on the containment barrier and located between inner and outer change rooms. | ○■ | ●■ | ●■ | ||
| 13) | A complete change of clothing is required prior to entering and exiting the containment area | ●■ | ●■ | |||
| 14) | On exit, a suit decontamination shower is provided on the containment barrier, between the containment area and the inner change room | ●■
Note. In the event of an emergency exit or failure of chemical shower, a system for decontaminating suit (e.g. gravity fed supply of chemical disinfectant) is needed Note: Suit Change and shower only required when lab operational Note: For BSL 4 suit lab, positive pressure suit with HEPA filtered breathing air must be worn | ||||
| 15) | Interlocked doors, if present, have manual overrides for emergency exit | ●■
|
●■ | ○□ | ○□ | |
| 16) | Exit from containment area provided with a walk-through compulsory shower on the containment barrier (i.e. between ‘dirty’ and ‘clean’ change anterooms)
|
○■
Note: Showers should be provided in the anteroom |
□ | ●■
Note: In BSL4 and ABSL4 cabinet lab, sequential passage through inner (dirty) changing area, personal shower and outer (clean) change room |
●■
Note: In BSL4 and ABSL4 suit lab, sequential passage through chemical shower, inner (dirty) change room, personal shower, and outer (clean) changing area. Chemical shower must be provided to decontaminate the surface of the suit before worker leaves the lab. In the event of an emergency exit or failure of chemical shower, a system for decontaminating suit (e.g. gravity fed supply of chemical disinfectant) is needed | |
| 17) | Entry to containment area provided via anteroom with sealed doors (e.g compression seal) | ●■ | ||||
| 18) | Entry to containment area provided via anteroom with airtight doors (e.g. inflatable or compression seal) | ●■ | ●■ | |||
| C. | SURFACE FINISHES AND FURNITURE: | |||||
| 1) | Doors, frames, casework and bench tops non-absorptive (organic materials avoided) | ●■
Note: Water resistant. |
●■
Note: Non-porous material. |
●■
Note: Non-porous material. | ||
| 2) | Interior surfaces cleanable and decontaminable | ●■ | ●■ | ●■ | ||
| 3) | Structural stability to withstand 1.25
times maximum design pressure under supply and exhaust fan failure conditions (i.e. no wall distortion or damage) |
●■ | ●■ | |||
| 4) | Floors slip resistant, impervious to liquids, and resistant to chemicals | ●■ | ●■ | ●■ | ||
| 5) | Penetrations in, floors, walls, and ceiling surfaces sealed | ○■ | ●■ | ●■ | ●■
Note: All penetrations in the internal shell of the lab, inner change room and suit storage room (if applicable) sealed | |
| 6) | Spaces around doors and ventilation openings capable of being sealed to facilitate safe and effective space decontamination or fumigation | ○□ | ●■ | ●■ | ●■ | |
| D. | HVAC: | |||||
| 1) | 100% outside air supplied | ○□
Note: Air may be decontaminated and recirculated to originating containment zone. |
■ | ■ | ●■ | |
| 2) | Directional inward air flow provided such that air will always flow towards areas of higher containment (e.g. ± 25Pa) | ●■ | ●■ | ●■ | ||
| 3) | The supply and exhaust ventilation systems shall maintain the containment area at negative pressure relative to surrounding areas and shall prevent outward airflow at any time. | ●■ | ●■ | ●■ | ||
| 4) | Visual pressure differential monitoring devices shall be provided at entry to containment area | ●■
Note: Locate device near clean change room |
●■
|
●■
Note: Locate device near clean change room | ||
| 5) | Room pressure differential monitoring
lines penetrating the containment barrier provided with filters of efficiency equal to that of EN 1822 H13 HEPA filtration |
●■ | ●■ | |||
| 6) | Alarm (visual or audible) provided in the containment area and outside the containment area (i.e. to warn others and maintenance personnel) to signal air handling systems failure | ●■ | ●■ | ●■ | ||
| 7) | Where deemed necessary by local risk assessment, supply air duct provided with back-draft protection (e.g. HEPA filter, bubble-tight backdraft damper) | ●■ | ●■ | |||
| 8) | Supply air to be HEPA filtered | ●■
Note: For BSL 4 cabinet labs - cabinet room, inner change room, and fumigation / decontamination chambers |
●■
Note: For BSL 4 suit labs - lab, decontamination shower Note: For ABSL 4 cabinet labs - cabinet room, inner change room, and fumigation / decontamination chambers Note: For ABSL 4 suit labs - lab, decontamination shower | |||
| 9) | Containment area supply air systems independent of other ventilation systems
|
○□
Note: Supply Air System can be combined with areas of lower containment when provided with back-draft protection (i.e. HEPA filter or bubble tight back draft damper) downstream from the connection |
●■
|
●■
Note: Dedicated non re-circulating ventilating system required for cabinet laboratory |
●■
Note: Labs with the same HVAC requirements (i.e. other BSL-4 labs, ABSL-4, BSL-3 Ag labs) may share ventilation systems if each individual laboratory system is isolated by gas tight dampers and HEPA filters
| |
| 10) | Dedicated breathing air supply system | ●■ | ||||
| 11) | N+1 Redundancy on supply fans | □ | ○□ | ○□ | ||
| 12) | N+1 Redundancy on exhaust fans | ●■ | ●■ | |||
| 13) | Supply air system interlocked (i.e. fans, dampers, electrical) with exhaust air system, to prevent sustained positive pressurization of containment area | ●■ | ●■ | ●■ | ||
| 14) | Exhaust air/gas HEPA filtered
|
○
Note: Option for exhaust air to be safely dispersed away from occupied areas and from building air intake locations only based on the agent summary statement and local risk assessment. |
●■
Note: Exhaust air must pass through HEPA filters before being discharged to the atmosphere without recirculation; exhaust capacity design should consider the heat and high moisture load produced during cleaning of animal rooms and cage wash process |
●■
Note: For BSL 4 cabinet labs - cabinet room, inner change room, and fumigation / decontamination chambers, room air exhaust through two HEPA filters in series before discharge to the outside |
●
Note: For BSL 4 suit labs - exhaust air from the lab, decontamination shower and fumigation or decontamination chambers must pass through two HEPA filters, in series, before discharge to the outside. Note: air may also be re-circulated after this filtration but only within the suit lab) | |
| 15) | Exhaust air filters arranged in series and parallel configuration. | ○□ | ||||
| 16) | Exhaust ventilation ducts should be fitted with arthropod-proof screens in arthropod facility | ■ | ■ | ■ | ||
| 17) | HEPA filters installed into the supply or exhaust air systems conform to the requirements of EN1822-1 or ISO 29463 | ●■ | ●■ | ●■ | ||
| 18) | Where HEPA filters are used for backdraft protection in accordance with local risk assessment, supply HEPA filter housings designed to withstand structural change at applied pressure of 2500Pa | ●■ | ●■ | ●■ | ||
| 19) | Any exhaust HEPA filter housings are designed to withstand structural change at applied pressure of 2500Pa and to be provided with a method of isolation and decontamination.
|
○□ | ●■ | ●■ | ●■ | |
| 20) | Containment area exhaust systems independent of other ventilation systems
|
●
Note: System can be combined with areas of lower containment when provided with HEPA filter upstream from the connection |
●■ | ●■ | ||
| 21) | Containment area exhaust air/gas must not re-circulate to any other area of the building and must be dispersed away from occupied areas and air intakes | ●■
or the exhaust must be HEPA-filtered |
●■
|
●■
| ||
| 22) | Exhaust air ductwork, flow control devices and duct sensors that are between the outside of the containment perimeter and HEPA filter or bubble tight back draft damper shall be sealed airtight in accordance with SMACNA Seal Class A2
|
●■ | ●■ | ●■ | ||
| 23) | Class III BSCs must be directly (hard) connected up through the second exhaust HEPA filter of the cabinet. Supply air must be provided in such a manner that prevents positive pressurization of the cabinet | ●■ | ●■ | N/A | ||
| 24) | Exhaust air from Class I or II BSCs, which will have been passed through HEPA filters, discharged in such a way as to avoid interference with the air balance of the cabinet or the building exhaust system | ●■ | N/A | ●■ | ||
| 25) | The HEPA filter housings should allow for safe leak testing of each filter and assembly | ○□ | ■ | ●■ | ||
| E. | CONTAINMENT PERIMETER: | |||||
| 1) | Double door barrier autoclave with a bio-seal located on containment barrier; body of autoclave preferably located outside of containment for ease of maintenance | ■ | ●■ | ●■ | ||
| 2) | Barrier autoclave equipped with interlocking doors, or visual or audible alarms to prevent both doors from being opened at the same time.
|
○■
Note: Autoclave on site preferably located in containment room and double ended |
N/A | N/A | ||
| 3) | Barrier autoclaves and fumigation chambers equipped with interlocking doors, and visual or audible alarms to prevent both doors from being opened at the same time. Interlock prevents opening of the outer door unless the autoclave has been operated through a decontamination cycle | ●■ | ●■ | |||
| 4) | Gas and liquid discharge from the autoclave chamber decontaminated | ○□ | ●■ | ●■ | ||
| 5) | For materials that cannot be autoclaved (e.g. heat sensitive equipment, samples, film) other proven technologies for waste treatment (e.g. incineration, chemical, or gas) provided at containment barrier | ○□ | ●■ | ●■ | ||
| 6) | All penetrations, conduits and wiring sealed with non-shrinking sealant at containment barrier | ○□ | ●■ | ●■ | ●■ | |
| 7) | Windows positioned on containment barrier sealed in place, window glazing material protected for required level of security | ●■ | ●■ | ●■ | ||
| 8) | Windows glass break-resistant | ○□ | ●■ | ●■ | ●■ | |
| 9) | Observation windows installed on containment barrier | ●■ | ●■ | ●■ | ||
| F. | LABORATORY SERVICES: | |||||
| 1) | Hand washing sinks located near the point of exit from the laboratory or in anteroom | ●■
Note: If the laboratory is segregated into different laboratories, a sink must also be available for hand washing in each zone |
●■
Note: In CL4 cabinet lab, sink provided near the door of the cabinet room(s), the inner change room and in the outer change room
|
N/A | ||
| 2) | Hand washing sinks provided with ‘hands-free’ capability | ●■ | ●■ | ●■ | ||
| 3) | All procedures involving the manipulation of infectious materials must be conducted within a BSC, or other physical containment devices | ○□
When a procedure cannot be performed within a Class II or III bio-safety cabinet, a combination of personal protective equipment and other containment devices must be used |
●■ | ●■
Note: Infected or potentially infected arthropods must be handled in BSCs or isolators; flying insects must be contained in double-netted cages |
○□
Note: When procedures cannot be performed in a BSC, alternate containment equipment should be used | |
| 4) | In a BSL 4 suit laboratory, all procedures conducted by personnel wearing a one-piece positive pressure suit ventilated with a life support system | ●■
Note: Breathing air systems must have redundant compressors, failure alarms and emergency backup | ||||
| 5) | BSCs and other primary containment devices provided | ●■ | ●■ | ●■ | ||
| 6) | BSCs installed so that fluctuations of the room air, supply air and exhaust air do not interfere with safe BSC operations | ●■ | ●■
|
●■
| ||
| 7) | BSCs located away from doors, heavily travelled laboratory areas, and other possible obstructions and airflow disruptions | ●■ | N/A | ●■ | ||
| 8) | Any Class III BSC has a pass-through dunk tank, fumigation chamber, or equivalent decontamination method. | ●■ | ●■ | |||
| 9) | Class III BSC has a HEPA filter on the supply air intake and two HEPA filters in series on the exhaust outlet of the unit. Gas tight dampers on the supply and exhaust ducts of the cabinet to permit gas or vapour decontamination of the unit. Ports for injection of test medium present on all HEPA filter housings | N/A | ●■ | ●■ | ||
| 10) | Class III BSC designed to permit maintenance and repairs of cabinet
mechanical systems (refrigeration, incubators, centrifuges, etc.) from the exterior of the cabinet whenever possible |
●■ | ●■ | |||
| 11) | Class III BSC operated at negative pressure to the surrounding containment area at all times | ●■ | ●■ | |||
| 12) | Equipment that may produce infectious aerosols are contained in devices that exhaust air through HEPA filtration before being discharged into the containment area. These HEPA filters should be tested and/or replaced at least annually | ●■
Note: Other equivalent technology accepted |
●■
Note: Other equivalent technology accepted | |||
| 13) | Emergency eyewash facilities provided in accordance with applicable regulations | ●■ | N/A | ●■ | ||
| 14) | Domestic water branch piping serving containment area areas provided with back flow prevention, and isolation valve located in close proximity to containment barrier | ○□ | ●■ | ●■ | ||
| 15) | Drain lines and associated piping
(including autoclave condensate) separated from areas of lower containment and connected to an effluent sterilization system |
●■ | ●■ | |||
| 16) | Liquid waste shall be decontaminated.
Any drain lines connected to effluent sterilization shall be sloped towards sterilization system to ensure gravity flow. Outside of containment perimeter, only closed connections are permissible. Design shall permit isolation and decontamination of piping for maintenance. |
○□
As determined by local risk assessment. |
●■
Effluents from Class III BSCs must be decontaminated before final discharge. Thermal treatment preferred |
●■
Effluents from the suit area, decontamination chamber, decontamination shower, or Class III BSCs must be decontaminated before final discharge. Thermal treatment preferred | ||
| 17) | Atmospheric venting systems provided with two HEPA filters in series and sealed up to the second filter | ●■ | ●■ | |||
| 18) | Compressed breathing air provided to positive-pressure personal protective equipment with hose connections provided in all areas where suits are worn, including chemical shower and suit change room. The system shall be equipped with breathing air compressors and back-up cylinders sufficient for 30 minutes per person. Breathing air shall be supplied at a suitable temperature and humidity for operator comfort.
|
●■ | ||||
| 19) | Life safety systems, lighting, HVAC systems, BSCs, security systems and other essential equipment supported with emergency back-up power | ●■ | ●■ | ●■ | ||
| 20) | Monitoring and control systems for air supply, exhaust, life support, alarms, entry and exit, and security systems on an uninterrupted power supply (UPS) | ●■ | ●■ | ●■ | ||
| 21) | Laboratory equipped with an audio and visual communication system between the containment area and outside support area | ○□ | ●■ | ●■ | ||
| 22) | Work area monitored (e.g. closed circuit TV) from outside laboratory perimeter (e.g. security/bio-safety office) | ●■ | ●■ | |||
| 23) | Used laboratory clothing treated as contaminated material and decontaminated before laundering | ●■ | ●■ | N/A | ||
| 24) | Infectious waste decontaminated before it is moved to other areas of the facility
Note: Method for decontaminating all infectious materials must be available effective and validated Note: All waste in arthropod facility should be decontaminated by autoclaving |
●■ | ●■ | ●■ | ||
| 25) | Animals infected with Risk Group 3 microorganisms housed in cages in
isolators or rooms with ventilation exhausts placed behind the cages |
■
Note: Risk of infectious aerosols can be reduced if animals are housed in containment caging systems. |
||||
| 26) | Safety mechanisms in place that prevent the cages and associated exhaust plenums from becoming positive to the surrounding area should the exhaust fan fail. The system should also be alarmed to indicate when operational malfunctions occur | ■ | ||||
| 27) | Housing areas for animals infected with Risk Group 4 agents maintain the criteria for containment described and applied for maximum containment laboratories (bio-safety Level 4) | □
Note: Animals should be housed in isolators
|
□
Note: Animals should be housed in isolators. *Large Animals infected with Risk Group 3 agents should be loose housed within primary containment precautions equivalent to BSCIII cabinet labs. | |||
| G. | SAFETY EQUIPMENT: | |||||
| 1) | Protective clothing with a solid front (such as tie-back or wrap around gowns, scrub suits, or coveralls) worn by workers when in the containment area | ●■ | ●■ | |||
| 2) | In BSL 4 suit laboratory, protective clothing such as scrub suits worn by workers before entering the room used for donning positive pressure suits | ●■ | ||||
| 3) | Protective clothing shall not be worn outside of the containment area. Reusable clothing shall be decontaminated with appropriate disinfectant before being laundered. Clothing and other personal protective equipment to be changed when contaminated | ●■ | ●■
Note: Protective clothing must be autoclaved after use |
●■
Note: Protective clothing must be autoclaved after use | ||
| 4) | Eye and face protection (goggles, mask, face shield or other splatter guard) used for anticipated splashes or sprays of infectious or other hazardous materials. Eye and face protection must be disposed of with other contaminated waste or decontaminated before reuse. Persons wearing contact lenses in containment areas must also wear eye protection.
Personal protective equipment to be changed when contaminated |
●■ | ○□
Note: Eye, face and respiratory protection as determined by the risk assessment |
N/A | ||
| 5) | Gloves must be worn to protect hands.
Glove selection based on an appropriate risk assessment. Alternatives to latex gloves should be available. Gloves must not be worn outside the containment area. In addition, gloves should be changed when contaminated, integrity compromised, or when otherwise necessary. Gloves removed and hands washed when work with hazardous materials completed and before leaving the containment area. Disposable gloves must not be washed or reused. Gloves must be disposed of with other contaminated waste |
●■ | ●■ | N/A | ||
Planning for Safety
- Door labelling should identify the type of lab and the responsible person including contact details
- A hand wash basin for hand washing must be included within each laboratory unless the environmental requirements of that laboratory prohibit it (ie aseptic areas). In special cases alternative hand sanitation measures can be provided although hand washing should not be precluded on leaving these laboratories. Hand wash basins should be fitted with stainless steel splash-backs fixed and sealed to the wall surface. Hand wash stations should also include a similarly fixed wall mounted mirror enabling the visual confirmation of the appropriate fitting of personal protective equipment.
- Laboratories hazards include:
- Hazardous biological agents
- Electrical fires due to the large amount of powered equipment
- Quantities of infectious substances
- Naked flames
- Quantities of combustible materials, e.g. paper and packaging.
- Slips and falls from spilled liquids on polished floor surfaces.
- In the event of an emergency, the laboratory may be unsafe to enter, therefore shut-off valves for gas and vacuum lines and electrical circuit breakers should be located outside the laboratory
- Plumbed or single use emergency eyewash stations should be provided in all laboratories.
- Emergency showers should be provided in all laboratories where a worker’s skin could become contaminated with infectious, skin irritant, toxic by skin absorption or corrosive materials.
- Eye wash stations and emergency shower locations should be identified with a highly visible signage. The floor immediately beneath the eyewash station and emergency shower should have a distinctive colour or pattern to a radius of about 1m to enable staff to find them easily when their vision may be temporarily impaired. These areas should be well illuminated.
- Emergency showers should provide an immediate high-volume output of water. Emergency showers should be fitted with a timed, automatic shut off valve. Floor drainage needs to be provided at the emergency shower. Floor and wall mounted electrical services should not be located near emergency showers without sufficient protection.
Maintenance
- Wherever possible, maintenance items should be placed outside of the laboratory such that maintenance staff needn’t enter and disrupt the laboratory during the course of their work. This provision includes for items in the ceiling void and especially refers to services not related to that laboratory.
- A provisional maintenance budget and plan should be established and presented for review at the conclusion of the facility design stage. This plan should include equipment stock, staffing and skills requirements. This plan should be checked and updated on conclusion of validation and handover.
Validation and Accreditation
Please help to expand this page. |
- SANAS accreditation SANS 14189 SAN17043 SANS 17025
- DTI Registration?
- R178
- Validation Checklist
PART C – Room Data Sheets
| Table 2: TYPICAL ACCOMMODATION SCHEDULE (400 BED HOSPITAL) | |||||
| Room Name | Service Description | Area
(m²) |
Min. Ceiling Height
(m) |
Occupancy
(Persons) |
Nominal Utilisation per day
(hours) |
| Community space | Reception and waiting areas | 2.7 | 8 | ||
| Male/Female/ Accessible Ablution areas | Ablution areas – male and female
One WHB per WC or urinal |
3+ (per cubicle) | 2.4 | 4 | |
| Lobby | Circulation | 2.7 | 4 | ||
| Changing room | Staff changing room - lockers to be provided | 2.4 | 2 | ||
| Tea Room | 2.7 | 2 | |||
| Storage space | Equipment and samples storage space
|
2.8 | 1 | 8 | |
| Administration | Daily administration functions | 2.8 | 8 | ||
| Pre-analytical /
Specimen Reception |
receive samples, capture and process test request and distribute the samples | 2.8 | 8 | ||
| Clinical –
Cyto Genetics |
Pathology | 2.8 | 8 | ||
| Clinical –
Molecular Biology |
Pathology | 2.8 | 8 | ||
| Clinical – Microbiology | Pathology | 2.8 | 8 | ||
| Clinical – Chemistry | Pathology | 2.8 | 8 | ||
| Clinical – Haematology | Pathology | 2.8 | 8 | ||
| Waste Management | Storage and treatment of waste | 2.8 | [TVR@2] | 4 | |
| Table 3: LABORATORY ROOM SERVICES | ||||||||||
| Room Name | Temp
(°C) |
Ventilation
(AC/h) |
Ventilation
Type |
Pressure Relative to
Ambient |
Wet Services | SSO
400V |
SSO
230V |
Task Illuminance
(lux) |
Data Points | Colour Rendering
(Ra) |
| Community spaces | 21-25 | 12 | Forced
Extraction |
= | 300 | - | ||||
| Ablution areas | 21-25 | 10 | Forced
Extraction |
- | WC
WHB |
300 | - | |||
| Lobby | N/A | 2 | Forced
Supply |
= | 300 | >80 | ||||
| Changing room | 21-25 | 2 | Forced
Supply |
= | WHB | 300 | - | |||
| Tea Room | 21-25 | 10 | Forced
Supply |
= | CW
HW |
300 | - | |||
| Storage space | 22-25 | 4-40 | Forced
Supply |
- | - | 300 | - | |||
| Administration | 21-25 | 2 | Forced
Supply |
= | - | 300/500 | >80 | |||
| Pre-analytical/
Specimen Reception |
21-25 | 12 | Supply/
Extraction |
= | WHB
EW |
500 | >90 | |||
| Clinical – Cyto Genetics | 21-25 | 6 | Supply/
Extraction |
- | WHB
EW |
500 | >90 | |||
| Clinical – Molecular Biology | 21-25 | 6 | Supply/
Extraction |
- | WHB
EW |
500 | >90 | |||
| Clinical – Microbiology | 21-25 | 6-20 | Supply/
Extraction |
+ | WHB
EW |
500 | >90 | |||
| Clinical – Chemistry | 21-25 | 6 | Supply/
Extraction |
- | WHB
ES, EW |
500 | >90 | |||
| Clinical – Haematology | 21-25 | 6 | Supply/
Extraction |
- | WHB
EW |
500 | >90 | |||
| Waste Management | 21-25 | 12 | Forced
Supply |
- | WHB
EW |
500 | [TVR@3] | - | ||
| Table 4 LABORATORY ROOM FINISHES | |||||
| Room Name | Floor
Material |
Wall / Floor Interface | Walls
Finish |
Ceiling
Finish |
Doors
Type |
| Community spaces | - | - | - | Acoustic ceiling board | Lockable solid door |
| Ablution areas | Tile | Skirting finished as per floor | Ceramic wall tiles with epoxy grout | Acoustic ceiling board | No requirement |
| Lobby | Tile | Skirting coved and finished as per floor | Washable acrylic paint | Acoustic ceiling board | No requirement |
| Changing room | Vinyl | Skirting coved and finished as per floor | Washable acrylic paint | Acoustic ceiling board | No requirement |
| Tea Room | Tile | Skirting coved and finished as per floor | Washable acrylic paint | Acoustic ceiling board | No requirement |
| Storage space | Jointless vinyl sheet | Skirting coved and finished as per floor | Washable acrylic paint | Washable acrylic paint | Lockable door |
| Administration | Tile | Skirting finished as per floor | Washable acrylic paint | Acoustic ceiling board | Lockable door |
| Pre-analytical/
Specimen Reception |
Jointless vinyl sheet | Skirting coved and finished as per floor | Washable acrylic paint | Vinyl clad ceiling tiles | Lockable door with door closer |
| Clinical – Cyto Genetics | Jointless vinyl sheet | Skirting coved and finished as per floor | Washable acrylic paint | Impervious monolithic, Washable acrylic paint | Lockable door with door closer |
| Clinical – Molecular Biology | Jointless vinyl sheet | Skirting coved and finished as per floor | Washable acrylic paint | Impervious monolithic, Washable | Lockable door with door closer |
| Clinical – Microbiology | Jointless vinyl sheet | Skirting coved and finished as per floor | Washable acrylic paint | Impervious monolithic, Washable | Lockable door with door closer |
| Clinical – Chemistry | Jointless vinyl sheet | Skirting coved and finished as per floor | Washable acrylic paint | Impervious monolithic, Washable | Lockable door with door closer |
| Clinical – Haematology | Jointless vinyl sheet | Skirting coved and finished as per floor | Washable acrylic paint | Impervious monolithic, Washable | Lockable door with door closer |
| Waste Management | Self- levelling epoxy | Skirting coved and finished as per floor | Washable acrylic paint | Impervious monolithic, Washable | Lockable door with door closer |
PART D – EXAMPLES
The following examples are provided courtesy of the National Health Laboratory Services.
Figure 3 Example of Central Lab - Vryheid
Figure 4 Example of Modular Lab
List of Abbreviations
| ACH | Air changes per hour |
| BSC | Biosafety cabinet |
| BSL1 | Biosafety level 1 |
| BSL2 | Biosafety level 2 |
| BSL3 | Biosafety level 3 |
| BSL4 | Biosafety level 4 |
| OH&S | Occupational Health and Safety |
| WHB | Wash Habd Basin |
| ES | Emergency Shower |
| EW | Eye Wash |
| WC | Toilet (Water Closet) |
| POCT | Point-of-care testing |
List of Definitions
| Acceptable Risk | Risks that have been reduced to a level that can be tolerated by the organisation having regard to its legal obligation and its own OH&S policy (SABS, 2011) |
| Anatomical Pathology | Involves the analysis of dead body tissues and cells (InformeDesign, 2005). |
| Biosafety code | A means of classifying human pathogens into categories according to the risk posed by that pathogen or a manipulation of that pathogen (National Department of Health, 2012). |
| Biosafety level 1 | A laboratory working with defined and characterised strains of viable infectious agents not known to cause disease or colonise in healthy human adults. However, these strains may be opportunistic and cause infection in the young, aged, and in immunocompromised or immunosuppressed individuals (National Department of Health, 2012). |
| Biosafety level 2 | A laboratory working with a broad spectrum of indigenous moderate-risk agents present in the community and associated with human disease of varying severity. Activities with low aerosol potential with these agents can be conducted on an open bench using good microbiological techniques. Procedures with high aerosol potential may increase the risk of exposure of personnel to infectious aerosols and must be conducted in primary containment equipment or devices (National Department of Health, 2012). |
| Biosafety level 3 | A laboratory working with indigenous or exotic agents which may readily cause serious and potentially lethal infections. Primary hazards to personnel working with these agents relate to auto-inoculation, ingestion and exposure to infectious aerosols (National Department of Health, 2012). |
| Clinical Laboratory | A laboratory for the biological, microbiological, immunohaematological, haematological, biophysical, cytological, pathological or other examination of materials derived from the human body for the purpose of providing information for the diagnosis, prevention and treatment of disease in, or assessment of the health of, human beings, and which may provide a consultant advisory service covering all aspects of laboratory investigation including the interpretation of results and advice on further appropriate investigation (SABS, 2009). |
| Clinical Pathology | Involves the analysis of bodily fluids, usually urine or blood (InformeDesign, 2005). Common clinical pathology diagnoses include blood cell counts, coagulation studies and blood glucose level determinants (InformeDesign, 2005). |
| Corrective Action | An action to eliminate the cause of a detected nonconformity or other undesirable situation (SABS, 2011). |
| Diagnostic specimen | Any human or animal material, which extends to excreta, secreta, blood and blood components, tissue or tissue fluids, that may be used for the purpose of diagnosis. This definition does not extend to live infected animals (National Department of Health, 2012). |
| Hazard | A source, situation or act with a potential for harm in terms of human injury or ill health, or a combination of these (SABS, 2011). |
| High containment laboratory | A laboratory which has special engineering features providing a safe environment for personnel to handle hazardous materials without posing danger to themselves, the community and environment (National Department of Health, 2012). These engineering features may extend to access control and special ventilation systems (National Department of Health, 2012). |
| Incident | Work related events in which an injury or ill health, regardless of severity, or fatality occurred, or could have occurred (SABS, 2011). |
| Laboratory Management | Person(s) who manage the activities of a laboratory headed by a laboratory director (SABS, 2009) |
| Medical Laboratory | See Clinical Laboratory |
| Microbiological laboratory | A laboratory which handles human pathogens capable of colonising in humans. It includes laboratories which handle infected or infested, or potentially infected or infested, indigenous vectors of human pathogens, or exotic vectors, irrespective if they are infected or infested (National Department of Health, 2012). |
| Nonconformity | Non-fulfilment of a requirement (SABS, 2011). |
| Occupational Health and Safety | Conditions and factors that affect, or could affect, the health and safety of employees or other workers (including temporary and contract workers), visitors, or any other person in the workplace (SABS, 2011). |
| Risk | Combination of the likelihood of an occurrence of a hazardous event or exposure and the severity of injury or ill health that can be caused by the event or exposure (SABS, 2011). |
| Workplace | Any physical location in which work related activities are performed under the control of the organisation (SABS, 2011). |
Acknowledgements
Special acknowledgement goes to the team responsible for researching and compiling this guidance document:
Etienne le Roux, NHLS;
Zibusiso Masuku, NHLS;
Faatiema Saalie, CSIR;
Tobias van Reenen, CSIR;
Hennie le Roux, NDoH,
Awaatief Railoun, NDoH
Bibliography
InformeDesign. (2005). Implications: Change in Clinical Labs in Hospitals.
National Department of Health. (2012). National Health Act 2003 - Regulations relating to the registration of microbiological laboratories and the acquisition, importation, handling, maintenance and supply of human pathogens.
SABS. (2009). SANS 15189:2009 Edition 2 Medical Laboratories - Particular requirements for quality and competence.
SABS. (2011). SANS 18001:2011 Occupational health and safety management systems - Requirements.
Stanford. (2011). Stanford Laboratory Standard & Design Guide : General Requirements for Stanford Laboratories. Version 2.0.
Wales Department of Health. (2006). HTM 05-03: Operational Provisions Part G: Laboratories on healthcare premises.
Welsh Health Estates. (2005). Health Building Note 15 – Facilities for Pathology Services. 2nd Edition.
[TVR@1]Please complete?
[TVR@2]Please complete sizes and occupancy
[TVR@3]Complete SSO & data
References
- ↑ 1.0 1.1 InformeDesign. (2005). Implications: Change in Clinical Labs in Hospitals
- ↑ SANS 15189:2009
- ↑ Stanford. (2011). Stanford Laboratory Standard & Design Guide : General Requirements for Stanford Laboratories. Version 2.0.
- ↑ SANS 10248 Management of healthcare waste (2004).|http://www.store.sabs.co.za/sans-10248-2004-ed-2-00
- ↑ SANS 10248-1 Management of healthcare waste - Part 1: Management of healthcare risk waste from a healthcare facility (2008)| |http://www.store.sabs.co.za/sans-10248-1-2008-ed-1-00
- ↑ 6.0 6.1 Welsh Health Estates, (2005)
- ↑ SABS. (2009). SANS 15189:2009 Edition 2 Medical Laboratories - Particular requirements for quality and competence.
- ↑ 8.0 8.1 Regulation R178 of the National Health Act of 2003[1]