Infrastructure Guidance for COVID-19/Alternate Care Sites: Difference between revisions
No edit summary |
|||
| Line 208: | Line 208: | ||
<div style="color:#000000;"><span style="background-color:#ffffff;">WHOWorld Health Organisation</span></div> | <div style="color:#000000;"><span style="background-color:#ffffff;">WHOWorld Health Organisation</span></div> | ||
Revision as of 13:36, 17 April 2020
Prepared for:
Business for South Africa
Contact person: Kate Roper
Tel: +27 #
Email: Kate.Roper@aurecongroup.com
|
Prepared by:
Infrastructure Innovation Research Group
Smart Places Cluster: CSIR
+27 (0)12 841 3007/ (0)82 574 3700
pdejager@csir.co.za
|
| DOCUMENT RETRIEVAL PAGE | |
| Report Title: | Minimum infrastructure requirements for Alternate Care Sites for SARS-CoV-2 |
| Authors: | Coralie van Reenen, Jako Nice, Peta de Jager and Toby van Reenen |
| Date: | 08 Apr 2020 |
| Project No.: | 60C4126 |
| Client Reference No.: | CDC-RFA-GH16-1644 |
| Abstract: | The global pandemic of COVID-19 caused by the coronavirus, SARS-CoV-2 is likely to result in a surge in need for medical care for Severe Acute Respiratory Syndrome (SARS) in South Africa. Considering the course of the pandemic in other countries, it is anticipated that South African hospitals will not have sufficient capacity to cope with the surge of persons requiring medical attention and that alternate care sites (ACS) will need to be established. These can be temporarily established in non-traditional environments, such as hotels, exhibition halls, community halls, and as field hospitals, on open spaces.
In the context of this document, a quarantine site is a facility for patients who do not require continuous professional medical care, while an ACS is defined as a temporary facility that can provide continuous medical care for SARS. This document provides principles and considerations, high-level guidance for minimum requirements and examples for ACS.
|
| Keyword(s): | Alternate Care Site, Field Hospital, COVID-19, Surge Capacity, Infrastructure, SARS |
| Competence Areas: | Smart Places |
Document roadmap
= Section one =
Purpose and approach
== Scope and assumptions ==
* suspected of having contracted SARS-CoV-2, (persons under investigation (PUIs)), who are symptomatic and/or are awaiting results,
- or are confirmed to be infected.
=== Exclusions ===
=== Service regime ===
* Temporary - limited to the part of the pandemic when the “conventional� hospital platform cannot meet demand. To be dismantled, thereafter.
- Uncomplicated, dedicated COVID-19 care. Patients with comorbidities, paediatrics will be prioritised for conventional facilities.
- 24 hour, 7 days a week operations.
Assumed mechanism of transmission
=== A call for strategic coordination ===
== Status quo ==
Rationale and transmission status
Table 1: Key clinical and infection control activities for different transmission scenarios[6]
| No Case | Sporadic Case | Clusters of Cases | Community Transmission | |
|---|---|---|---|---|
| Faculty Space, Including for Transmission | Usual Space. Enhanced Screening and triage at all points of first access to the health system | Dedicated COVID-19 patient care areas within health facility (e.g. infectious disease ward, isolation rooms in emergency or ICU wards). | More patient care areas re-purposed for COVID-19 within the health system, especially for severe cases | Expanded care for severe cases in new hospitals or temporary hospital facilities |
| Staff | Usual space. Enhanced screening and triage at all points of first access to the health system | Dedicated COVID-19 patient care areas within health facility (e.g. infectious disease ward, isolation rooms in emergency or ICU wards) | More patient care areas repurposed for COVID-19 within the health system, especially for severe cases | Expanded care for severe cases in new hospitals or temporary hospital facilities |
| Supplies | * On-hand supplies. Equip wards for COVID-19 treatment.
|
* Expanded inventory of supplies with detailed protocols for use.
|
* Conservation, adaptation, selected re-use when safe.
|
* Activate contingency planning should critical equipment be in short supply.
|
| Standard of Care | Usual care with enhanced awareness and recognition of immediate needs for first COVID-19 patients | Usual care and treatment for all patients, including those with COVID-19 | Identify context-relevant core services. Shift service delivery platforms. Consider reduction in elective patient encounters, including elective surgical procedures. | Mass critical care (e.g. open ICU for cohorted patients). |
| Care areas expansion | No requirements for expansion | Designate 10 beds per suspected COVID-19 case | Expand COVID-19 patientcare areas by a factor of 35 | Expand COVID-19 patient care areas by a factor of 58 |
Quantification of need
* ACS should be preferably identified with space for expansion. The set-up should be done so that levels of care can be upgraded to higher levels of care.
- This guidance makes the assumption that only uncomplicated COVID-19 cases will be treated at an ACS, entailing that patients with comorbidities, and paediatrics will be referred to conventional facilities. Depending on epidemic trajectory, it may be necessary to expand services to include a greater range of clinical services at ACS.
Strategic approach
Figure 1: WHO Strategic approach clinical care.
* Within and around existing healthcare facilities, via reconfiguration and/or augmentation.
- In existing non-healthcare buildings suitable for repurposing, such as universities, hotels and conference centres, warehouses, gyms, hostels etc.
- On open fields, including paved parking areas with rapidly constructed, dismountable structures, such as modular tented structures or using rapid modular construction techniques.
=
Section two =
Typology dictates
Table 2: SARS ACS precedents
| Site type:
Typological response: Service model: Precedent:
|
Existing hospital
Minor adaptive reuse Clustered cohort Sung-Shan Military Hospital Taipei[7]
|
Conversion of existing non-isolation buildings to isolation wards for treatment of SARS patients. Steps for conversion and implementation described. Nosocomial infection rate 0.6% ascribed to non-compliance with procedures.
|
Infrastructure steps taken: 1) Clear buildings of people & equipment. 2) Fans (commercial grade 3X1m blaes, 65W, 60Hz) above each window. 3) plug doors to create negative pressure relative to corridor (0.028-0.07 water gauge in rooms to 0.0 in corridors.) 4) Close stairways between floors. 5) creating three zones at the ground floor for entry A: clean zone for changing and administration; B: Intermediate zone for removing inner layer of PPE, showering; C: contaminated zone for removing outer layer of PPE; 6) cleaning regime described. 7) Patient transport described; 8) Treatment of SARS patients and handling of equipment described: Interesting: Centralize facilities to better control / train health care workers and nosocomial infections. |
|
Site type: Typological response: Service model: Precedent:
|
Existing hospital Augmentation Mass ICU A medical tent is stationed outside Richmond University Medical Center in West Brighton[8].
|
File:Picture 55.png | |
| Site type:
Typological response: Service model: Precedent:
|
Existing hotel
Adaptive reuse Obligate - Cellular/ single room Theory only…[9]
|
File:Picture 47.png
| |
|
Site type: Typological response: Service model: Precedent:
|
Conference centre Repurposing Mass ICU NHS Nightingale Hospital London[10] Javits Center, New York[11] Los Angeles Convention Centre
|
|
|
| File:Picture 479.png
| |||
|
Site type: Typological response: Service model: Precedents:
|
Open field Modular construction Cellular/ single room Volumetric Building Companies (VBC) Philadelphia[12] (Linear format) MAII – USA[13] (Clustered configuration)
|
|
File:Image26.png.png |
|
Site type: Typological response: Service model: Precedent: |
Open field Repurposed shipping containers Mass ICU CURA, Milan
|
File:Image5.png.png
|
|
|
Site type: Typological response: Service model: Precedent:
|
Open field Tented structure Mass ICU Central Park, New Y[14] |
File:Picture 52.png | |
No site is likely to meet all requirements and recommendations set out in this document. Adaptations and compromises will be necessary. The examples set out above demonstrate that a variety of host settings are workable, provided that the appropriate utility can be contrived.
== ACS Planning Team ==
* Disaster response / emergency management coordination,
- Clinical care and staffing,
- Facility set-up, operations and management,
- Security,
- Transport (patient, staff),
- Engineering and project management,
- Procurement and coordination of supplies, equipment and pharmaceuticals, and
- Community liaison to ensure
that concerns of the adjacent population on understood an addressed.
== Site selection ==
* 100 Bed ACS/ hospital conversion, requires +- 4 300 m2
- 1000 Bed ACS/ hospital conversion, requires +- 17 600 m2
=== Criteria ===
- Affordability (costs, including operational costs known and budget identified),
- Sufficient physical space and capacity to house the immediate need, with the potential to accommodate physical space requirements. For example, open site solutions should not be sloping,
- Legal rights and encumbrances, including renewal opportunity,
- Free from clear and present danger,
- Outside attenuation zones, floodplains,
- Outside high wind zones,
- Structure in good repair,
- Access to sufficient capacity for
- potable water,
- adequate drainage,
- telephone and/or wifi,
- electricity, and
- Likelihood of acceptance of hosting an ACS by the adjacent and local community.
Desirable
- A zone for cleaning, disinfection, and decontamination of equipment at least 15 metres away from occupied areas with access to water, a hard impervious surface and drying areas in the sun, with runoff discharge into the sewer and not into marine ecosystems or the environment.
- Capacity for expansion.
- Accessible to at least two roadways to provide continued access in the event that one roadway becomes blocked on inaccessible.
Infection prevention and control
=== Transmission-based precautions ===
=== Standard precautions ===
As SARS-CoV-2 is not considered airborne, respiratory protection against airborne transmission is not considered necessary, except where aerosolisation of particles may be a risk.
According to CDC * tracheal intubation,
According to doctors in the field also when performing * COVID-19 diagnostic sampling as patients can be induced to cough and sneeze.
|
=== Spatial strategies for infection prevention and control ===
Restricted access and zone control
==== Site layout and master-planning ====
Figure 2: Layout for a SARS facility, clustering functions with minimised cross-over [15]
Figure 3: Tygerberg Hospital virus triage unit site layout [16]
Figure 4: Patient cohorting strategy[17]==== Cohorting ====
==== Workflow ====
Figure 5: Workflow in small unit [18]
Figure 6: COVID19 contact spread infection prevention and control recommend flow diagram
Figure 7: Workflow in large unit[19]
=== Operational strategies ===
Cleaning, disinfection and decontamination
* Cleaning with detergent and water.
- Disinfection with 75% alcohol solution (metal surfaces).
- Sodium hypochlorite (1,000 ppm)/ Household bleach.
- [about:blank Disinfectants listed on the EPA][20] (for non-critical environmental cleaning).
- High intensity ultraviolet surface disinfection (UV-C).
- Decontamination and sterilisation of clinical equipment.
Goods and waste management
* The National and Provincial Health Care Risk Waste Management Regulations.
- National Department of Health COVID-19 Environmental Health Guidelines[21].
==== Materials and finishes ====
* level,
- free of dust and oil,
- impervious and smooth,
- slip-resistant in wet areas (e.g. patient ablutions).
=== Personal protection ===
Hand sanitation
Figure 8: Clinical hand wash basin[22]
Figure 9: Portable hand wash basins can be provided in ACS [23]
==== Personal protective equipment ====
=== General transmission mitigation ===
Water and sanitation
Airborne precautions
== Structural integrity and operational responsibility ==
== Decommissioning and closure ==
* conduct a site walkthrough with the facility owner when shutdown activities are completed to ensure that removal of equipment and supplies, cleaning and other surge closure activities have been completed to the owner’s satisfaction.
- perform medical records storage procedures.
== Health, safety and well-being ==
=== General provisions ===
- Minimised and controlled entry and exit points, with suitable control.
- Clearly identified, accessible and marked routes for patients, staff, goods and waste.
- Clear designation of restricted zones.
Site level provisions
- Safe staff parking and arrival of staff via planned and public transport.
- Clearly demarcated parking for people with disabilities.
- Arrival and departure point for patients via public transport, passenger vehicles, and emergency service.
- Supply of goods and removal of waste.
- Limited safe visitor parking.
Within and between buildings
- Clear entrances.
- Routes free of all hazards, for example, rubbish bins.
- All clinical, patient and support areas to be accessible by trolley.
=== Signage ===
* Clearly visible, simple font, font size, contrasting colours, placed in field of vision
- Washable
- Comprehensive safety signage - fire signage (exits, equipment etc.)
- Restricted areas clearly marked
- Identification signage - each patient space to be allocated a unique number and a whiteboard or perspex sheet for writing the patient’s name
=== Safety and security ===
Figure 10: Zonal approach to security[27]
=== Comfort and dignity ===
Figure 11: Transparent barrier for observation with canvas blinds for patient privacy and separation [29]== Schedule of accommodation ==
* Laboratory services
- Catering
- Laundry
- CSSD
- Maintenance and cleaning
- Mortuary
= Section
three =
Clinical services
Triage
=== Inpatient ACS accommodation ===
* suspected, unconfirmed cases, under observation (PUIs), to be accommodated in isolation facilities (separate rooms, if possible);
- patients with confirmed COVID-19 with mild to moderate disease, not requiring dedicated oxygen therapy;
- patients who require dedicated oxygen therapy;
- patients requiring mechanical ventilation; and
- recovered/ confirmed negative.
Protective isolation facilities
Figure 12: COVID-19 ACS - protective isolation – bed layout
Figure 13: COVID-19 ACS – mild/ moderate patient bed layout
Figure 14: COVID-19 ACS – mild/ moderate patient shared ward layout
Figure 15: COVID-19 ACS – severe/critical patient shared ward layout
Patient services
* Repaired and refurbished beds from condemned hospital stocks.
- South African National Standard, SANS 521:2013 Edition 3.5, on Hospital beds and cots ISBN 978-0-626-28830-3.
- Beds listed on the National Treasury (See Appendix E).
Table 3:Patient services
| Service/ Capacity | Triage | Isolation | Mild – moderate inpatient | Severe case wards | Critical case wards | |
|---|---|---|---|---|---|---|
| Power – 16A 230V Single socket outlet | As needed | 1 per bed | 1 per bed | 3 per bed | 6 per bed | |
| UPS Power – 16A 230V Single socket outlet | As needed | 1 per bed | 1 per bed | 1 per bed | 2 per bed | |
| Medical Air* (LP)400kPa | No | Yes | No | Yes | Yes | |
| Medical O2
400kPa |
Portable/shared | Portable/shared | No | One | Two | |
| Vacuum
-40kPa |
No | Portable/shared | style="border:0.5pt solid #000000;padding-top:0cm;padding-bottom:0cm;padding-left:0.191cm;padding-right:0.191cm;" | Portable/shared | Yes | Yes |
| Equipment rail | Yes | Yes | ||||
| Upper room UVGI | Optional | Optional | Optional | |||
| Examination light | No | No | Yes | Yes | Yes | |
| Ventilation rate | 60 L/s per person | 10 L/s per person | 10 L/s per person | 10 L/s per person | 12 ACH | |
| Notes:
*Mobile units recommended for intermittent use. 3 per 20 beds ** There are some ventilators which have built-in compressors allowing them to function without Medical Air. This is however, not the norm. With Ventilators probably being the most difficult medical device to obtain at present, it would be prudent to rather allow for Medical Air at each bed. | ||||||
* Electrocardiograph (ECG): Could be omitted if monitors have full 12 lead ECG function.
- Blood gas analyser: Could be omitted if a Lab Services are available.
Patient ablutions
* 1 toilet for every 8 persons.
- 1 shower for every 8 persons.
- 1 disabled ablution for every 8 regular ablutions (or part thereof).
- 1 disabled shower for every 8 persons (or part thereof).
==== Makeshift sluice areas ====
==== Dedicated patient treatment areas ====
* Counselling and consulting room (can be shared), as.shown in Figure 16.
- Minor procedures room, as per the example provided in Figure 17.
Figure 16: Consulting room example layout
Figure 17: Treatment/ minor procedures room example layout== Logistical services ==
=== Visitors entry point ===
* In paediatric wards, one parent may be accommodated to visit a patient. In such cases, direct access for the visitor should be provided so that the visitor does not need to pass through the general patient area. Appropriate PPE must be donned before entering the patient area and hand washing/sanitising must be done when exiting the area.
- Non-patients who are accompanying suspected patients to the facility for testing or admission must be accommodated in a well-ventilated, spacious waiting area. Signage in such waiting areas must inform visitors about symptoms, hand hygiene and PPE.
- Hand washing/sanitizing facilities.
=== Staff areas ===
Staff change rooms
==== Staff rest areas ====
==== Staff auxiliary services ====
Figure 18: Example of overnight sleeping area for staff
=== Bulk storage ===
Support services
Workflow principle
Figure 19: Linen processing cycle[30] === Laboratory ===
Figure 20: Example of modular laboratory
Pharmacy
Radiology
Laundry services
==== Siting and model selection considerations ====
* Water and power capacity.
- Ease of access to the ACS’s main corridors and internal transport routes.
- The noise factor of the facility and its impact on nearby patient care departments.
* Delivery areas to allow sufficient space to ensure that vehicles can manoeuvre and park easily at the reception and dispatch bays.
- Access to the ACS service roads and public roads.
Functional requirements
=== Catering services ===
=== CSSD ===
Figure 21: An example of a small CSSD facility[34]
Maintenance and cleaning
Mortuary services
==== Location and layout of mortuary service ====
==== Sizing of mortuary ====
==== Services ====
* Hygienic floor drains that are resistant to corrosion from blood and chlorine should be provided in all “wet areas� of the mortuary and should be directly connected to the sewer system. These areas include body preparation, autopsy space, etc. These areas require thorough cleaning after every procedure, using large quantities of water and decontaminating and disinfecting chemicals and soaps.
- Sluicing facilities are to be provided in both the body-preparation and autopsy areas if they are not a common area.
- Open floor channels should be avoided. Where this is not possible, these should be covered by durable, flush-fitted stainless steel grids.
- No sewer connections external to the mortuary services should be made to the line between the wet area drains and the main sewer system in order to prevent backflow to other areas.
- The provision of hot and cold water in the facility is imperative, with all basins, sinks, ablution areas and autopsy tables being provided with both.
- Anti-backflow devices should be fitted to the water-supply lines serving mortuary table faucets to prevent backflow should supply water pressure fail.
- Electricity supply to the mortuary – particularly for refrigeration purposes – is to be provided from the essential supply system for the hospital. Alternatively, a back-up generator is to be supplied to allow for the maintenance of required temperatures in the cooling/freezing facilities in the mortuary.
Finishes
= Section four =
Environmental controls
General indoor environment conditions
* Systems should be set to maximise the introduction of fresh air and maintain the pressure regime (see ventilation).
- The following internal temperature range should be maintained 19 - 24oC.
- Cooling systems should be able to cater for projected internal heat gains from people, lighting and equipment. Indicative heat gains in
- treatment areas are 8W/m2 from people, 15W/m2 from lighting and 3 W/m2 equipment and
- In critical care areas 16W/m2 from people, 15W/m2 from lighting and 60W/m2 equipment.
- As heat gain can vary widely between items of equipment, heat gain and utilisation rates for equipment should be obtained from the manufacturer to establish this more accurately.
Solid waste from ACS
* HCRW is segregated at the point of generation and shall be containerized to minimize the risk of contamination.
- Waste generated from patients in isolation or quarantine in a designated facility health facility, is treated as health care risk waste (HCRW) as per SANS 10248-1-2008.
- The HCRW is properly packaged in sealed, leak and puncture proof containers/ boxes.
- The HCRW is labelled with the bio- hazard symbol/ sign and marked “Corona virus or COVID-19�.
- The HCRW is stored separately from other waste generated.
- The collection, transportation, treatment and disposal is provided by only an appointed/ appropriate contractor/ service provider, however, ensure that waste is safely stored until the health care waste management company can pick it up and that the company knows and acknowledges that waste was generated by suspected or confirmed COVID-19.
- The waste management company collecting must ensure that and treated and disposal is conducted at license waste treatment/ disposal facilities .
- All personnel or staff in contact with patients must be geared with appreciate personal protective equipment (PPE’s) at all times to prevent exposure or risk to health.
- Monitoring should be done at such facilities.
- All, bags, bins and boxes must be adequately sealed, as not to leak any fluids, and must be wiped down with 0.05% chlorine solution.
* Develop a waste management plan following national guidelines and best practice standards for the disposal of medical waste (WHO, 2020).
- Establish procedures with medical waste service providers to regularly pick up the waste and dispose of this safely.
- Provision should be made for 5kg of solid waste per bed per day and this should be monitored and supplemented where it appears this may be inadequate.
- Ensure that access to waste is secure and controlled, for instance, by using lockable waste 1000l containers kept in a location that can only be accessed by health facility and nominated service delivery staff.
- Vermin control programs must be implemented throughout the site with HCRW collection points prioritised
- Provision for safe cleaning and disinfection of containers should be provided.
- Waste must not be allowed to accumulate or be stored inappropriate or unsecured containers.
Engineering services
* IUSS Building Engineering Services[39].
- NHS Nightingale Instruction Manual [40].
=== Ventilation ===
* Mechanical systems should be set to maximise fresh air supply to the facility. There should be no recirculated air without HEPA filtration or other validated decontamination process.
- A pressure regime should be established, as shown in figure 2, to 'push' air from clean areas, to dirty areas and then out of the building.
- A clean air supply of over 10 L/s per person should be targeted for odour control.
- Fresh air supply shall not be located near patient beds to avoid drafts in winter.
- Extraction points can be located near patient beds in isolation wards or at high level in long stay wards. Short circuiting of air between high level supply and extraction is a performance risk in winter.
- Noise from ventilation systems and fans shall be below 45 dBA
- Protected lobbies, internal partitions, door arrangements, fans and extracts should be used to maintain the pressure regime and airflow as indicated in Figure 21 below.
Figure 21: Ventilation in temporary facilities [41]=== Electrical power ===
Sufficient and reliable power must be available at the facility for envisaged medical equipment, medical gases, lighting and ventilation equipment. Power installations for the temporary facility can be divided into three zones as indicated below. These are existing services, the temporary service zone and services in each bay.
The following should be considered by a competent engineering professional.=== Existing services ===
- Capacity: Evaluate whether sufficient power to accommodate envisaged medical equipment, additional lighting and heating, ventilation and air conditioning can be provided. If existing capacity is insufficient, investigate if it is possible to route additional power from additional locations/transformers around the site or from adjacent sites.
- Safety: The existing electrical distribution network must be able to supply the required equipment load. If this is insufficient/appears unreliable, identify how this can be supported.
- Resilience: Evaluate back-up power and a UPS capacity against essential services demand. If existing capacity is not sufficient, source and establish temporary service capacity.
Temporary service zones
- Identify locations for temporary service zones where equipment can be located.
- Ensure that equipment and maintenance access is safe and easy.
- Ensure that all distribution boards, circuit breakers and cables are clearly labelled.
Services in each bay
- Provide pre-wired power strips / trunking as per bay requirements.
- Check that these include sufficient plug points for envisaged equipment.
- Ensure that trunking will carry required equipment loadings. The IUSS Building Engineering Services Guide can be used to check requirements[42].
Figure 22: Layout of power in a temporary installation[43]=== Water ===
==== Supply ====
* Storage 25 L per bed.
- Consumption 180 L/bed.day W/O laundry; 250L /bed. day W laundry.
Hand washing
See infection control for clinical wash-hand basins==== Showers ====
=== Medical gases, oxygen and vacuum (suction) ===
Figure 23: Medical gas service layout[44]=== Lighting ===
* Lighting levels should be provided in line with the indoor lighting levels recommended in the Table 6 of IUSS Building Engineering Services [45].
- Mobile task lighting systems may be adopted in the serious and critical stay wards to supplement incorrect lighting quality.
- Emergency lighting and illuminated emergency egress signage should be linked to the back-up power system.
- External security lighting in external parking areas and spaces around the building should be enhanced to ensure the security of medical staff who need to change shifts at night.
=== Fire safety ===
A functional fire alarm system should be available to support the patient care setting. Fire is a very real threat due to the possibility of an oxygen enriched atmosphere developing so ventilation is crucial.
* Patients may have a very high dependency.
- Areas are not specifically designed for patients and do not meet guidance on fire compartmentation and progressive horizontal evacuation.
- Large numbers of patients supplied with oxygen up to 10 litres per minute.
- Possibility of oxygen concentrations exceeding those generally found in the atmosphere- less risk if effective ventilation or large volume i.e. high ceilings.
- Staff who may not normally work together
- Staff who may not be familiar with the area
- Staff not trained in fire safety, progressive horizontal evacuation or oxygen isolation for the specific area.
* An automatic fire detection system
- An emergency egress plans are prepared that include patients who have a very high dependency.
- Signage, notices and lighting are installed and are working effectively.
- Management processes are in place to minimise the risk of fire from ignition sources, fuels and oxygen.
- Staff are trained and that fire safety guide sheet for staff is developed and issued.
- Emergency egress routes are kept clear.
- AIA - Knowledge Net . (2020). Academy of Architecture for Health. Retrieved April 7, 2020, from AIA- Knowledge Net: https://network.aia.org/communities/community-home/digestviewer?communitykey=5ac54771-1122-4d1f-ac18-d2d12d6a94fb&tab=digestviewer
- ASHRAE. (2020, March 31). COVID-19 (Coronavirus) Preparedness Resources. Retrieved April 8, 2020, from ASHRAE: https://www.ashrae.org/technical-resources/resources
- Barbara Bannister, V. P. (2009, January ). Framework for the design and operation of high-level isolation units: consensus of the European Network of Infectious Diseases. Retrieved April 8, 2020, from Lancet- Infectious Diseases : https://www.thelancet.com/journals/laninf/article/PIIS1473309908703049/fulltext
- BBC. (2020, March 31). Coronavirus: Building NHS Nightingale Hospital London. Retrieved April 7, 2020, from BBC News : https://www.bbc.com/news/in-pictures-52092253
- BDP, 2020. NHS nightingale instruction manual. Available at: http://www.bdp.com/globalassets/projects/nhs-nightingale-hospital/nhs-nightingale-instruction-manual.pdf [Accessed 7/4/2020].
- Beirne, M. (2020, March 30). Modular Builders Mobilize to Deliver Prefab Modules for Coronavirus Care. Retrieved April 13, 2020, from Probuilder: https://www.probuilder.com/modular-builders-mobilize-deliver-prefab-modules-coronavirus-care
- CDC- Center for Disease Control and Prevention. (2020, April 6). Alternate Care Sites. Retrieved April 7, 2020, from CDC - Coronavirus Disease 2019 (COVID-19): https://www.cdc.gov/coronavirus/2019-ncov/hcp/alternative-care-sites.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcoronavirus%2F2019-ncov%2Fhealthcare-facilities%2Falternative-care-sites.html
- CIBSE, 2020. CIBSE - Chartered Institution of Building Services Engineers. Available at: https://www.cibse.org/ [Accessed 8/4/2020].
- European Centre for Disease Prevention and Control . (2020, February 26). Checklist for hospitals preparing for the reception and care of coronavirus 2019 (COVID-19) patients. Retrieved April 8, 2020, from European Centre for Disease Prevention and Control : https://www.ecdc.europa.eu/en/publications-data/checklist-hospitals-preparing-reception-and-care-coronavirus-2019-COVID-19
- European Centre for Disease Prevention and Control. (2020, April 7). Publications and Data . Retrieved April 8, 2020, from European Centre for Disease Prevention and Control: https://www.ecdc.europa.eu/en/publications-data/checklist-hospitals-preparing-reception-and-care-coronavirus-2019-COVID-19
- Federal Healthcare Resilience Task Force . (2020, March 30). Alternate Care Site (ACS) Toolkit First Edition. Retrieved April 11, 2020, from https://files.asprtracie.hhs.gov/documents/acs-toolkit-ed1-20200330-1022.pdf
- Fung, C., Hsieh, T., Tan, K., Loh, C., Wu, J., Li, C., . . . Lee, C. (2004). Rapid Creation of a Temporary Isolation Ward for Patients With Severe Acute Respiratory Syndrome in Taiwan. Infection Control & Hospital Epidemiology, 25(12), 1026-1032. doi:10.1086/502339.
- Health Systems Research Inc. (2005, April). Altered Standards of Care in Mass Casualty Events. Retrieved April 13, 2020, from https://asprtracie.hhs.gov/technical-resources/resource/491/altered-standards-of-care-in-mass-casualty-events
- Health Systems Research, Inc. (2007, February). Mass Medical Care with Scarce Resources. Retrieved April 13, 2020, from A Community Planning Guide: https://www.calhospitalprepare.org/sites/main/files/resources/Mass%20Medical%20Care%20with%20Scarce%20Resources.pdf
- Institute of Medicine. (2010). Medical Surge Capacity: Workshop Summary (2010). Washington, DC: The National Academies Press.
- IUSS. 2014a. Inclusive Environments. Retrieved 16 April, 2020 from https://www.iussonline.co.za/norms-standards/healthcare-environment/34-inclusive-environments
- IUSS. 2014b. Security. Retrieved 16 April, 2020 from https://www.iussonline.co.za/norms-standards/healthcare-environment/40-security
- IUSS. 2014c. Laundry and linen department. Retrieved 16 April, 2020 from https://www.iussonline.co.za/norms-standards/support-services/30-laundry-and-linen-department
- IUSS. 2014d. Catering services. https://www.iussonline.co.za/norms-standards/support-services/23-catering-services-for-hospitals
- IUSS.2014e. Central Sterile Supply Department. Retrieved 16 April, 2020 from https://www.iussonline.co.za/norms-standards/support-services/24-central-sterile-service-department
- IUSS. (2017, January 23). Building Engineering Services. Retrieved April 8, 2020, from Improving South Africa's Healthcare Infrastructure: https://www.iussonline.co.za/norms-standards/healthcare-environment/60-building-engineering-servi
- Keane, K. (2020, April 02). These Architects Are Addressing COVID-19 Health Care Infrastructure Capacity. Retrieved April 02, 2020, from Architect: https://www.architectmagazine.com/practice/these-architects-are-addressing-COVID-19-health-care-infrastructure-capacity_o
- Ministry of Health- Singapore . (2019, December 27). Being Prepared for a Pandemic. Retrieved April 8, 2020, from Ministry of Health- Singapore: https://www.moh.gov.sg/diseases-updates/being-prepared-for-a-pandemic
- National Department of Health South Africa . (2020, March). Covid- 19 Enviromental Health Guidelines . Retrieved April 13, 2020, from https://www.nicd.ac.za/wp-content/uploads/2020/03/COVID-19-ENVIRONMENTAL-HEALTH-GUIDELINE-1.pdf
- New Zealand Government . (2020, April 8). Key documents and legislation. Retrieved April 8, 2020, from Unite against COVID-19 : https://COVID19.govt.nz/resources/key-documents-and-legislation/
- NHS, 2020a. C0131 COVID-19 estates facilities fire risk assessment. Available at: https://www.england.nhs.uk/coronavirus/wp-content/uploads/sites/52/2020/03/C0131-COVID-19-estates-facilities-fire-risk-assessment.pdf [Accessed 8/4/2020].
- NHS. (2020, April)b. Nightingale Cubicle Beds. Retrieved April 13, 2020, from Archpaper: https://cdn.archpaper.com/wp-content/uploads/2020/04/NHS-Nightingale-Cubicle-Beds-2-copy-scaled.jpg
- Shroer, J. (2020, April 3). What if...we used a hotel for patients? Retrieved 9 April, 2020, form ASHE https://www.ashe.org/what-if-we-used-hotel-patients
- Steyn, M. (2020, April 8). Summary notes of the International Water Association (IWA) Webinar: “COVID-19: A Water Professional’s Perspective�.
- United States Environmental Protection Agency. (2020, April 9). List N: Disinfectants for Use Against SARS-CoV-2. Retrieved April 9, 2020, from Pesticide Registration: https://www.epa.gov/pesticide-registration/list-n-disinfectants-use-against-sars-cov-2
- van Reenen, T., Singh, T., Poluta, M. de Jager, P. 2019. Implementation of upper room UVGI - an abridged guide. Retrieved April 16, 2020 from https://www.tb-ipcp.co.za/tools-resources/uvgi-documents/national-guidelines-abridged
- Williams, A. (2020, March 23). Shipping container-based ICU being developed for COVID-19 treatment. Retrieved April 13, 2020, from New Atlas: https://newatlas.com/architecture/carlo-ratti-cura-shipping-containers/
- WHO. (n.d.). Setting up an Ebola Treatment Centre (ETC). Retrieved April 8, 2020, from https://extranet.who.int/ebolafmt/sites/default/files/ETC_considerations_for_set_up.pdf
- WHO. (2020). Hospital readiness checklist for COVID-19. Retrieved April 8, 2020, from World Health Organization- Regional Office for Europe : http://www.euro.who.int/en/health-topics/health-emergencies/coronavirus-COVID-19/novel-coronavirus-2019-ncov-technical-guidance/coronavirus-disease-COVID-19-outbreak-technical-guidance-europe/hospital-readiness-checklist-for-COVID-19
- WHO. (2020a). Operational considerations for case management of COVID-19 in health facility and community. Retrieved April 8, 2020, from Interim guidance: https://apps.who.int/iris/handle/10665/331492
- WHO. (2020b). Severe Acute Respiratory Infections Treatment Centre. Geneva, Switzerland : WHO- World Health Organization .
- WHO, (2020c). Safe Management of Waste from Health Care Activities. Available at: https://apps.who.int/iris/bitstream/handle/10665/85349/9789241548564_eng.pdf;jsessionid=92BF582C7F14F2BE80E4987A5DAA7840?sequence=1 [Accessed 9/4/2020].
- Wong, E. (2020, March 24). TB, HIV and COVID-19: urgent questions as three epidemics collide. Retrieved April 8, 2020, from The Conversation : https://theconversation.com/tb-hiv-and-COVID-19-urgent-questions-as-three-epidemics-collide-134554
== Minimum requirements for temporary COVID Response healthcare facilities : decision tree ==
Appendix B: Summary notes of the International Water Association (IWA) Webinar: “COVID-19: A Water Professional’s Perspective�
Appendix C: Examples of accommodation schedule for isolation ward
Patient spaces (based on work by Edwina Fleming)
| Patient Spaces | ||
| Room type | General description | Spatial requirement |
| Ward room | 1 bed | # All non-essential furniture to be removed for infection prevention and control purposes
|
| Bathroom | Toilet, shower/bath, basin | # Single use bathroom is recommended, not communal.
|
| If rooms have access to external balcony, access can be granted, however if room balconies are adjoined, access must be restricted. | ||
| If room have access to external garden, this must be restricted, unless external patio can be cordoned off. | ||
Service spaces for isolation ward
| Shared Spaces | ||
| Room type | General description | Spatial and other requirement |
| Linen store | General cupboard or room utilised for controlled storage and distribution of clean linen. | # Must be once of decontaminated before use.
|
| Surgical store | General cupboard or room utilised for controlled storage and distribution of surgical items. This room can be combined with the temporary medicine store. | # Must be once of decontaminated before use.
|
| Medicine store | General cupboard utilised for controlled storage and distribution of medication, can be shared with surgical store. See above note | |
style="background-color:transparent;border-top:1pt
solid #000000;border-bottom:1pt solid #000000;border-left:1.5pt solid #000000;border-right:1pt solid #000000;padding:0.176cm;color:#000000;" | Dirty linen room |
General cupboard or room utilised for controlled storage of dirty/contaminated linen. Used linen to be stored in bags and bagged into waste bag for transport to laundry | # Must be once of decontaminated before use.
|
| Body hold room | In the event that a suspected patient becomes ill and dies prior to being transferred to a hospital site, a holding room is required for the body. This is an open room, preferably no windows and controlled access. | # Must be once of decontaminated before use.
|
| No shared meeting or socialising area to be provided | ||
| No shared dining area to be provided for patients, in room dining only | ||
Appendix D: Examples of accommodation schedule for ward for mild to severe cases
Patient spaces (based on work by Edwina Fleming)
| Patient Spaces | ||
| Room type | General description | Spatial requirement |
| Ward room ‘Mild & Moderate’ acuity | Large multi-bed ward.
|
# Side cupboard
|
| Ward room ‘Critical & Severe’ acuity | Large multi-bed ward up. | # Side cupboard
|
| Ward room Recovery | Large multi-bed ward. | *
|
| Bathroom | Toilet, shower/bath, basin | *
|
Standard bed service required per bed
Nurse call One per bed
Task light One per bed
| ||
| If rooms have access to external balcony, access can be granted, however if room balconies are adjoined, access must be restricted. | ||
| If room has access to external garden, this must be restricted, unless external patio can be cordoned off. | ||
Service spaces (based on work by Edwina Fleming)
| Shared Spaces | ||
| Room type | General description | style="background-color:transparent;border-top:1.5pt solid #000000;border-bottom:1pt solid #000000;border-left:none;border-right:1.5pt
solid #000000;padding:0.176cm;color:#000000;" | Spatial and other requirement |
| Utilities | ||
| Linen store | Room utilised for controlled storage and distribution of clean linen. | *
|
Clean utility
Surgical store
Medicine stores
|
Separate/ combined rooms to be utilised for controlled storage. Lockable.
General cupboard utilised for controlled storage and distribution of medication, can be shared with surgical store. See above note
|
*
|
| Housekeepers store | *
| |
| Dirty linen/utility room | Room utilised for controlled storage of dirty/contaminated linen. Used linen to be stored in bags and bagged into waste bag for transport to laundry. Wash hand basin. | *
|
| Body hold room | Room utilised for the deceased patients, prior to collection by mortuary. | *
|
| Equipment store | *
| |
| Dirty utility/ waste combined | Storage and handling of waste, prior to collection | *
|
| Nurse station and records | Nurse record keeping and | *
|
| Room type | General description | Spatial and other requirement |
| Clinical | ||
| Consultation/counselling room/ can be dual function | For patient follow up and minor treatment not performed at bed side. | *
|
| Emergency procedure room | For minor procedures that do not require theatre – Operating theatres are not provided at ACS sites | *
|
| Laboratory room | style="background-color:transparent;border-top:none;border-bottom:1.5pt
solid #000000;border-left:none;border-right:1pt solid #000000;padding:0.176cm;color:#000000;" | Room utilised for analysing samples in the GeneXpert, storage of samples, and data capturing. Autoclaves may be provided. |
*
|
| Room type | General description | Spatial and other requirement |
| Access | ||
| Donning area for staff | Entrance room into the facility, for all staff donning | *
|
| Doffing area for staff | Exit room from the facility, for all staff doffing | *
|
| Trolley wash area | Trolley wash area | *
|
| Wheelchair and porters | Storage area for distribution of wheel chairs to patients | *
|
| Room type | General description | Spatial and other requirement |
| Staff | ||
| Staff change room | Central between the entrance and exit room (Refer to donning and doffing area) | *
|
| Staff rest rooms | *
|
*
|
| Room type | *
|
*
|
| Public | ||
| 24 Hour Help Desk | Basic information and public | *
|
style="background-color:transparent;border-top:none;border-bottom:1pt solid #000000;border-left:1.5pt solid
#000000;border-right:1pt solid #000000;padding:0.176cm;color:#000000;" | External waiting area |
Waiting area for parents of ill children, and caretaker of elderly | *
|
| Public Toilets | For waiting parent or caregiver only | *
|
| No shared meeting or socialising area to be provided | ||
| No shared dining area to be provided for patients, in room dining only | ||
| Support Services | |||||||||||||||||||
| Room type | General description | Spatial requirement | |||||||||||||||||
| Central Sterilise Service Department (CSSD)
| |||||||||||||||||||
|
Total area required inclusive of circulation: 110 m2 | ||||||||||||||||||
| This is short-term temporary or mobile assembly requirement. Service required: water, electricity and sewer holding tank | |||||||||||||||||||
| Room type | General description | Spatial requirement | |||||||||||||||||
| Diagnostics (Radiology fixed and mobile) | |||||||||||||||||||
|
Total area required inclusive of circulation: 95 m2 | ||||||||||||||||||
| This is short-term temporary or mobile assembly requirement. Service required water, electricity. | |||||||||||||||||||
| Room type | General description | Spatial requirement | |||||||||||||||||
| Pharmacy (discharge dispensing and bulk storage) | |||||||||||||||||||
|
Total area required inclusive of circulation: 280 m2 | ||||||||||||||||||
| This is short-term temporary or mobile assembly requirement. Service required water, electricity and sewer holding tank. This is medicine storage and ward distribution, the only dispensing that will occur, is for discharged patients. | |||||||||||||||||||
| Room type | General description | Spatial requirement | |||||||||||||||||
| Laboratory services (testing and data capture) | |||||||||||||||||||
|
Total area required inclusive of circulation: 37 m2 | ||||||||||||||||||
| This is short-term temporary or mobile assembly requirement. Service required water, electricity and sewer holding tank. This is a testing, and data capture local site service – supported by NHLS, | |||||||||||||||||||
| Room type | General description | Spatial requirement | |||||||||||||||||
| Administration | |||||||||||||||||||
|
Total area required inclusive of circulation: 127 m2 | ||||||||||||||||||
This is short-term temporary or mobile assembly requirement. Service required water, electricity. This
is only essential administration. | |||||||||||||||||||
| Room type | General description | Spatial requirement | |||||||||||||||||
| Bulk stores (all supplies) | |||||||||||||||||||
|
Total area required inclusive of circulation: 180 m2 | ||||||||||||||||||
| This is short-term temporary or mobile assembly requirement. Service required water, electricity. This is bulk storage for all goods and the asset management and distribution thereof. | |||||||||||||||||||
| Room type | General description | Spatial requirement | |||||||||||||||||
| Mortuary short term hold (Viewing included) | |||||||||||||||||||
|
Total area required inclusive of circulation: 148 m2
| ||||||||||||||||||
| This is short-term temporary or mobile assembly requirement. Service required water, electricity and sewer holding tank. This unit will not be freezing bodies, only refrigeration will be provided. Local mortuary services will be involved to ensure at 24hr service turnaround time, in line with Health ministers directive (08-04-2020). | |||||||||||||||||||
| Room type | General description | Spatial requirement | |||||||||||||||||
| Laundry, outsourced service model (Holding with basic sluicing only) | |||||||||||||||||||
|
Total area required inclusive of circulation: 184 m2 | ||||||||||||||||||
| This is short-term temporary or mobile assembly requirement. Service required water, electricity. This only a holding site, with outsourced local contractors as per local Health Department procurement | |||||||||||||||||||
| Room type | General description | Spatial requirement | |||||||||||||||||
| Kitchen, outsourced service model (Receive and Dispatch only) | |||||||||||||||||||
|
Total area required inclusive of circulation: 100 m2 | ||||||||||||||||||
| This is short-term temporary or mobile assembly requirement. Service required water, electricity. This only a holding and supply site, with outsourced local contractors as per local Health Department procurement | |||||||||||||||||||
| Room type | General description | Spatial requirement | |||||||||||||||||
| Engineering services and temporary plant | |||||||||||||||||||
|
Total area required inclusive of circulation: 440 m2 | ||||||||||||||||||
| This is short-term temporary or mobile assembly requirement. Provision of all essential series and short bulk connection to all municipal service. | |||||||||||||||||||
| Room type | General description | Spatial requirement | |||||||||||||||||
| Waste management, outsourced service model (Holding only) | |||||||||||||||||||
ding-right:0.191cm;"
|
Total area required inclusive of circulation: 149 m2 | ||||||||||||||||||
| This is short-term temporary or mobile assembly requirement. Service required water, electricity. This only a holding site, with outsourced local contractors as per local Health Department procurement | |||||||||||||||||||
Appendix E: Hospital bed specifications
| Bed, hospital, two section with TrendelenbergTo comply with the specifications in Appendix A, SEE ATTACHED.Note: where the item offered differs from the specification in Appendix A, except for the items specified below, the supplier must indicate the deviation and supply relevant detailsMust comply with IEC 60601-2-52 (Particular requirements for basic safety and essential performance of medical beds) paragraphs: 201.1, 201.3, 201.7, 201.9, 201.13, 201.15, annex BB and annex CCMild steel frame with epoxy/nylon powder-coated finish to comply with SANS 778 paragraph 5.2, proof of compliance must be submitted.Epoxy/nylon powder coating colours: white, cream or greyBed must support a patient mass of 180 kgAdjustable backrest with gas spring assist, suitable for 100 kg patientTo be fitted with castors, two swivel , two lockingCastors must comply with the latest issue of SANS 621, proof of compliance must be submitted. Where castors are fitted into steel tubular legs, the tube shall be of wall thickness not less than 2,0mm and castors shall be fixed to the tube by one of the following methods: a. Solid plug (long) complying with SANS 621 subsection 3.5.6 orb. Screwed into 35mm long sleeves welded into the tubular members and locked in an acceptable mannerc. Rubber or plastic expanding sleeves for fitting castors are not acceptableRemovable head and foot ends (ABS material may be offered)With collapsible safety sides, to comply with IEC 60601-2-52Mattress support: mattress support other than weldmesh is required, provide detailsThe following accessories must be accommodated to fit on the bed. The price for these accessories must not be included on this item bid price. a. Driprodb. Patient lifting pole with chain or strap and handle, must support a mass of 75 kgc. Traction pole with pulleys and weightsd. Bedding support according to specification in Appendix A, subsection 3.14Item to be evaluated as series with item RT24-02-003, |
| Bed, hospital high-lowTo comply with the latest issue of CKS 447Note: where the item offered differs from the specification in CKS 447, except for the items specified below, the supplier must indicate the deviation and supply relevant detailsMust comply with IEC 60601-2-52 (Particular requirements for basic safety and essential performance of medical beds) paragraphs: 201.1, 201.3, 201.7, 201.9, 201.13, 201.15, annex BB and annex CCMild steel frame with epoxy/nylon powder-coated finish to comply with SANS 778 paragraph 5.2, proof of compliance must be submitted.Epoxy/nylon powder coating colours: white, cream or greyBed must support a patient mass of 180 kgHydraulically operated variable height operated by dual sided foot pedalsAdjustable backrest with gas spring assist, suitable for 100 kg patientTo be fitted with castors with a central castor locking systemCastors must comply with the latest issue of SANS 621, proof of compliance must be submitted. Where castors are fitted into steel tubular legs, the tube shall be of wall thickness not less than 2,0mm Removable head and foot ends (ABS material may be offered)With collapsible safety sides, to comply with IEC 60601-2-52Mattress support: mattress support other than weldmesh is required, provide detailsThe following accessories must be accommodated to fit on the bed. The price for these accessories must not be included on this item bid price. a. Driprodb. Patient lifting pole with chain or strap and handle, must support a mass of 75 kgc. Traction pole with pulleys and weightsd. Bedding support according to specification in Appendix A, subsection 3.14 |
| Bed, hospital intensive care, 4 sectionTo comply with the latest issue of CKS 447 Note: where the item offered differs from the specification in CKS 447, except for the items specified below, the supplier must indicate the deviation and supply relevant detailsThe mattress platform shall be in four sections allowing for a profiling actionMust comply with IEC 60601-2-52 (Particular requirements for basic safety and essential performance of medical beds) paragraphs: 201.1, 201.3, 201.7, 201.9, 201.13, 201.15, annex BB and annex CCMild steel frame with epoxy/nylon powder-coated finish to comply with SANS 778 paragraph 5.2, proof of compliance must be submitted.Epoxy/nylon powder coating colours: white, cream or greyThe bed shall have a four section platform. The knee-break section adjustable via a manual mechanismBed must support a patient mass of 180 kgHydraulically operated variable height operated by dual sided foot pedalsAdjustable backrest with gas spring assist, suitable for 100 kg patientTo be fitted with castors with a central castor locking systemCastors must comply with the latest issue of SANS 621, proof of compliance must be submitted. Where castors are fitted into steel tubular legs, the tube shall be of wall thickness not less than 2,0mmRemovable head and foot ends (ABS material may be offered)With collapsible safety sides, to comply with IEC 60601-2-52Mattress support: mattress support other than weldmesh is required, provide detailsOxygen cylinder holderExtension of bed must comply to CKS 447, subsection 3.5 The following accessories MUST be offered to fit on the bed. The price for these accessories must not be included on this item bid price. a. Driprodb. Patient lifting pole with chain or strap and handle, must support a mass of 75 kgc. Traction pole with pulleys and weights |
Bed, hospital, obstetric, high-low, tilting, 2 section, complete with mattressBed and fittings must accommodate various labour and delivery positionsMust comply with IEC 60601-2-52 (Particular requirements for basic safety and essential performance of medical beds) paragraphs: 201.1, 201.3, 201.7, 201.9, 201.13, 201.15, annex BB and annex CCMild steel frame with epoxy/nylon powder-coated finish to comply with SANS 778 paragraph 5.2, proof of compliance must be submitted.Epoxy/nylon powder coating colours: white, cream or greyMattress platform material Bed must support a patient
mass of 180 kgRemovable leg sectionAdjustable backrest with gas spring assist (0 to 60 degrees) with quick release. Controls at both sides of bedHydraulically operated variable height operated by dual sided foot pedalsHeight range (mattress platform): 500 to 750 mm (approximately)Trendelenberg tilt, 12 degreesTo be fitted with 125 mm castors with a central castor locking systemCastors must comply with the latest issue of SANS 621, proof of compliance must be submitted. Where castors are fitted into steel tubular legs, the tube shall be of wall thickness not less than 2,0mmWith collapsible safety sides, to comply with IEC 60601-2-52Rubber buffer wheels at the corners of bed head endMattress, two section, for body and foot sections, with cover. Body section must be suitable for all profile anglesMattress to comply with the latest issue of SANS 640 AND 1291-1 (type 2), except for thickness, as below, proof of compliance must be submitted.Mattress must support a patient of at least 180 kg and return to original shape when not in useTo be constructed of flexible polyurethane foam complying with class 30, grade no.12 of SANS 640Thickness: 150 mm (-0 and +5mm)Manufacturer must supply a 5 year warranty on the mattressTwo lithotomy poles, height adjustable, swivel action, with leg support (not straps)Douche fittingDouche tray, stainless steelDrip ro |
| Cot, adult, complete with mattressTo comply with the latest issue SANS 521, subsection 5.4, fig. 5Note: where the item offered differs from the specification in SANS 521, except for the items specified below, the supplier must indicate the deviation and supply relevant detailsMust comply with IEC 60601-2-52 (Particular requirements for basic safety and essential performance of medical beds) paragraphs: 201.1, 201.3, 201.7, 201.9, 201.13, 201.15, annex BB and annex CCMild steel frame with epoxy/nylon powder-coated finish to comply with SANS 778 paragraph 5.2, proof of compliance must be submitted.Epoxy/nylon powder coating colours: white, cream or greyLength: 2 045 mm (± 12 mm)Width: 915 mm (± 6 mm)Mattress support: mattress support other than weldmesh is required, provide detailsMattress must comply with specifications in item RT24-02-014Item to be evaluated as series with item RT24-02-010, RT24-02-014 and RT24-02-015 in terms of paragraph 16.4 in the Special Conditions of Contract. |
| Cot, adult, with rising backrest and Trendelenberg, with mattressTo comply with the latest issue SANS 521, subsection 5.4, fig. 5Note: where the item offered differs from the specification in SANS 521, except for the items specified below, the supplier must indicate the deviation and supply relevant detailsMust comply with IEC 60601-2-52 (Particular requirements for basic safety and essential performance of medical beds) paragraphs: 201.1, 201.3, 201.7, 201.9, 201.13, 201.15, annex BB and annex CCMild steel frame with epoxy/nylon powder-coated finish to comply with SANS 778 paragraph 5.2, proof of compliance must be submitted.Epoxy/nylon powder coating colours: white, cream or greyRising backrest support.Trendelenberg and anti-Trendelenberg positionsLength: 2 045 mm (± 12 mm)Width: 915 mm (± 6 mm)Mattress support: mattress support other than weldmesh is required, provide detailsMattress must comply with specifications in item RT24-02-014 |
Appendix F: Example healthcare technology
| Bed (see appendix E) and pressure reducing mattress |
| Ventilator (with humidifier) |
| Multi-parameter Patient Monitor |
| Infusion Pump: The standard would be 4 Volumetric Pumps and 2 syringe drivers per bed but the exact requirement needs to be specified by the clinicians depending on their treatment protocol |
| Drip Stand |
| Wall suction unit |
| Stethoscope |
| Ambubag adult (Resuscitator) |
| O2 Flowmeters |
Appendix G: Example crash cart healthcare technology
(Courtesy REAF Consulting)
Defibrillator
Mobile suction machine
ENT set
Laryngoscope with blades size 1,2,3,4 straight and curved
Ambubag adult
Ambubag pads
Ambubag neonatal
Oxygen gauge
Infrared Thermometer
Plaster Scissors
Forceps, Artery, Straight, 20cm
Forceps, Magills, 20cm
Video Laryngoscope (Difficult intubation)
Detector, Oesophageal Intubation (difficult intubation)
Inflator, Tracheal Tube Cuff
Disposable, consumable and drugs needs to be added.
Appendix H: WHO diagnostic equipment list
Lab screening test kit
Lab confirmation test kit
RT-PCR kit
Extraction kit
Cartridges for RT-PCR automatic systems
Swab and Viral transport medium
- ↑ https://thehillside.info/index.php?title=Infrastructure_Guidance_for_COVID-19/Alternate_Care_Sites
- ↑ Health Systems Research Inc., 2005
- ↑ IUSS, 2017
- ↑ WHO, 2020 a
- ↑ [https://theconversation.com/tb-hiv-and-covid-19-urgent-questions-as-three-epidemics-collide-134554 The Conversation, 2020]
- ↑ WHO, 2020a
- ↑ Fung et al, 2004
- ↑ Joseph Ostapiuk, 2020
- ↑ Salus, 2020, Shroer, 2020
- ↑ BBC News, 2020
- ↑ Katherine Keane, 2020
- ↑ Beirne, 2020
- ↑ Courtesy Philip Patrick Sun
- ↑ NBC news, 2020
- ↑ WHO: 2020 b
- ↑ Western Cape Provincial Government, 2020
- ↑ WHO: 2020 b
- ↑ Western Cape Provincial Government, 2020
- ↑ Western Cape Provincial Government, 2020
- ↑ United States Environmental Protection Agency, 2020
- ↑ National Department of Health South Africa, 2020
- ↑ De Jager, 2020
- ↑ BDP, 2020
- ↑ National Department of Health South Africa, 2020
- ↑ [../../../../../../../../../C:%5CUsers%5CPdeJager%5CDesktop%5CTobias%20van%20Reenen1,%20Tanusha%20Singh2 Van Reenen et al, 2019]
- ↑ IUSS, 2014a
- ↑ IUSS, 2014b
- ↑ IUSS, 2014b
- ↑ WHO, 2020b, p.49 and NHS, 2020b
- ↑ IUSS, 2014c
- ↑ NHS, 2020
- ↑ IUSS, 2014c
- ↑ IUSS, 2014d
- ↑ IUSS, 2014e
- ↑ National Department of Health South Africa, 2020
- ↑ Zhejiang University, 2020
- ↑ WHO, 24 March 2020
- ↑ National Department of Health South Africa, 2020
- ↑ IUSS, 2017
- ↑ BDP, 2020
- ↑ BDP, 2020
- ↑ IUSS, 2017
- ↑ BDP, 2020
- ↑ BDP, 2020
- ↑ IUSS, 2017
Infrastructure Minimum Guidelines for Alternate Care Sites for COVID-19
This guidance work was initiated under project titled:
Reducing Nosocomial and Community-Acquired Tuberculosis by Strengthening the Capacity of the South African Department of Health to Improve Implementation of Infection Control and Waste Management at All Levels of the Health System Under the President's Emergency Plan for AIDS Relief (PEPFAR)
Purpose and approach
The outbreak of Covid-19 in South Africa is likely to result in a surge in need for medical care. Considering the course of the pandemic in other countries, it is anticipated that South African hospitals will not have sufficient capacity to cope with the surge of persons requiring medical attention and that isolation sites and alternate care sites (ACS) will need to be established. These can be established in non-traditional environments, such as hotels, exhibition/community halls, and temporary field hospitals. An isolation site is a facility for patients who do not require medical care, while an ACS is defined as a temporary facility that can provide medical care for Severe Acute Respiratory Syndrome (the degree of care will depend on the need). This document provides principles and considerations, high level guidance for minimum requirements and examples. While an extensive set of health facility guidelines does exist (see http://www.iuss.co.za), these are applicable for conventional facilities and thus include services and guidelines that are not necessarily relevant to the treatment of Covid-19, specifically, nor for the rapid and temporary establishment of facilities. The CSIR responded rapidly to the invitation extended by BSA, to formulate high-level guidance through consultation and research. The team reached out to professional industry bodies for inputs, in particular the South African Institute for Architects (SAIA), The Gauteng Institute for Architects (GiFA) and the South African Federation of Hospital Engineering (SAFHE), by inviting input via a 36 hour research charette. Relevant historical and contemporary literature was consulted, precedents identified and critically reviewed. Material from the IUSS, international literature and guidance and input gathered from the broader architectural, engineering and healthcare professional communities was synthesised and moderated by the CSIR team. The draft was reviewed by an expert review panel. Contributors and reviewers are acknowledged in text.
Scope and assumptions
ACSs as discussed in this document are dedicated, temporary facilities for identification and treatment of persons: suspected of having contracted SARS-CoV-2, (persons under investigation (PUIs)), who are symptomatic and/or are awaiting results or are confirmed to be infected ACS will accommodate a variety of clinical, logistical, support and auxiliary services associated with the render of care. Pediatric patients are to be accommodated in separate wards, where strictly controlled visitation may be allowed.
Exclusions:
Quarantine facilities - for asymptomatic persons who are in self- or imposed isolation, but not displaying symptoms, or who are symptomatic, but are able to safely recover without clinical intervention are not considered in this document.. Service regime: The following assumptions are made with respect to services under consideration. Temporary - limited to the part of the pandemic when the “conventional” hospital platform cannot meet demand. To be dismantled, thereafter. Uncomplicated, dedicated Covid-19 care. Patients with comorbidities, paediatrics will be prioritised for conventional facilities. 24 hour, 7 days a week operations Assumed mechanism of transmission transmission is understood to be preferentially by the contact and droplet routes with opportunistic airborne transmission in special circumstances. reclassification of transmission mechanisms may nullify some of the approaches presented in this guidance.
Rationale and need
According to the WHO, Based on the largest cohort of Covid-19 patients, about 40% of patients with Covid-19 may have mild disease, where treatment is mostly symptomatic and does not require inpatient care; about 40% of patients have moderate disease that may require inpatient care; 15% of patients will have severe disease that requires oxygen therapy or other inpatient interventions; and about 5% have critical disease that requires mechanical ventilation. However, the evolution of the outbreak in some countries has shown a higher proportion of severe and critical cases and the need to rapidly increase surge capacity to prevent rapid exhaustion of biomedical supplies and staff. In some countries, doubling rates of cases every three days has been observed. South Africa has a high burden of disease, with a high prevalence of HIV and TB. Although evidence is yet to emerge of the effect of SARS-CoV-2 on a population with these pre-existing conditions, there is reason to proceed with caution. There is potential direct and indirect benefit of ACS to people living with HIV and TB, as well as to general public health and the health system preservation. It appears that South Africa is on the cusp between cluster transmission and community transmission according to WHO’s classification, indicating that preparation can include temporary hospital facilities and mass critical care.
| No Case | Sporadic Case | Clusters of Cases | Community Transmission | |
|---|---|---|---|---|
| Faculty Space, Including for Transmission | Usual Space. Enhanced Screening and triage at all points of first access to the health system | Dedicated COVID-19 patient care areas within health facility (e.g. infectious disease ward, isolation rooms in emergency or ICU wards). | More patient care areas re-purposed for COVID-19 within the health system, especially for severe cases | Expanded care for severe cases in new hospitals or temporary hospital facilities |
| Staff | Usual space. Enhanced screening and triage at all points of first access to the health system | Dedicated COVID-19 patient care areas within health facility (e.g. infectious disease ward, isolation rooms in emergency or ICU wards) | More patient care areas repurposed for COVID-19 within the health system, especially for severe cases | Expanded care for severe cases in new hospitals or temporary hospital facilities |
| Supplies |
|
|
|
|
| Standard of Care | Usual care with enhanced awareness and recognition of immediate needs for first COVID-19 patients | Usual care and treatment for all patients, including those with COVID-19 | Identify context-relevant core services. Shift service delivery platforms. Consider reduction in elective patient encounters, including elective surgical procedures. | Mass critical care (e.g. open ICU for cohorted patients). |
| Care areas expansion | No requirements for expansion | Designate 10 beds per suspected COVID-19 case | Expand COVID-19 patientcare areas by a factor of 35 | Expand COVID-19 patient care areas by a factor of 58 |
Quantification of need
AAt this time there are various parallel initiatives aimed at forecasting the South African epidemic, quantifying the projected need for facilities, and shortfall in existing capacity. At this time, there is no consensus on this. This section will be updated as further data becomes available.
ACS will attend to mild to moderately affected COVID-19 patients where basic, targeted medical care will be provided. Should patients’ needs evolve, requiring escalation of care, then transfer of patients from ACS sites to conventional sites of care will be needed as a matter of course, bringing with it logistical challenges and risks. The following pragmatic approach, aligned with the WHO recommended strategic approach, is suggested.
- ACS should be preferably identified with space for expansion. The set-up should be done so that levels of care can be upgraded to higher levels of care.
- This guidance makes the assumption that only uncomplicated COVID-19 cases will be treated at an ACS, entailing that patients with comorbidities, and paediatrics will be referred to conventional facilities. Depending on epidemic trajectory, it may be necessary to expand services to include a greater range of clinical services at ACS
Strategic approach
According to WHO, for clinical care, six major interventions must be put into place immediately, and then scaled up according to epidemiologic scenarios.
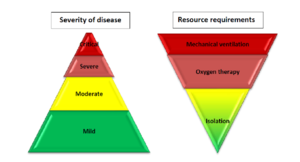
To meet the requirements set out above, prospective sites should be evaluated, by scrutinizing plans, satellite images and by physical inspection (walkabout). Expanded, services, under the current State of Disaster, could, on a temporary basis be hosted:
- Within and around existing healthcare facilities, via reconfiguration and/or augmentation.
- In existing non-healthcare buildings suitable for repurposing, such as universities, hotels and conference centres, warehouses, gyms etc.
- On open fields, including paved parking areas with rapidly constructed, dismantlable structures, such as modular tented structures or using rapid modular construction techniques.
The type of “host” site selected will strongly influence or dictate the choice of ACS service model. Some typological responses and service model are set out below in precedent examples.
| Case severity, risk factors* | Recommendations |
|---|---|
| Mild | Patient should be instructed to self-isolate and contact COVID-19 information line for advice on testing
and referral. |
| Moderate, with no risk factors | Test suspected COVID-19 cases according to diagnostic strategy. Isolation/ cohorting in:
(i.e. adjacent COVID-19 designated health post/EMT-type 1, telemedicine)
|
| Moderate, with risk factors | Patient should be instructed to self-isolate and call COVID-19 hotline for emergency referral as soon as possible |
| Severe | Hospitalization for isolation (or cohorting) and inpatient treatment.
|
| Critical | Hospitalization for isolation (or cohorting) and inpatient treatment.
|
* Known risk factors for severe COVID-19: age over 60 years, hypertension, diabetes, cardiovascular disease, chronic respiratory
disease, immunocompromising conditions.
Note: Probable cases should be retested immediately.
No site is likely to meet all requirements and recommendations set out in this document, Adaptations and compromises will be necessary. The examples set out above demonstrate that a variety of host settings are workable, provided that the appropriate utility can be contrived.
Ideally all services should be provided on site. However, the use of off-site services is not unconventional and may be practical/feasible for temporary sites, provided suitable procedures are followed. It should be noted that the key limitations are to be found in resource constraints (staff, equipment, funding), and therefore coordinated options appraisal and prioritisation is needed.
Infection Prevention and Control
Guidance for COVID-19 Infection Prevention and Control can be accessed Here



