Ventilation and COVID-19: Difference between revisions
→Transmission routes: removed fomite from preferential route list |
|||
| (56 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
[[Category:COVID-19]] | [[Category:COVID-19]] | ||
[[Category:Crosscutting Issues]] | [[Category:Crosscutting Issues]] | ||
== Context == | ==Context== | ||
This article aims to to contextualize COVID-19 related ventilation guidelines in a field of developing clinical evidence. This is done with the hope of empowering the reader to scrutinize proposed interventions within this context and employ appropriate and efficient solutions. The information and guidance in this article is the developing opinion of the author and does not represent any regulatory or institutional mandate or authority. The evidence supporting this opinion is evolving and therefore the opinion is similarly subject to change. The reader is encouraged to return to this article frequently to review any changes or updates. | This article aims to to contextualize COVID-19 related ventilation guidelines in a field of developing clinical evidence. This is done with the hope of empowering the reader to scrutinize proposed interventions within this context and employ appropriate and efficient solutions. The information and guidance in this article is the developing opinion of the author and does not represent any regulatory or institutional mandate or authority. The evidence supporting this opinion is evolving and therefore the opinion is similarly subject to change. The reader is encouraged to return to this article frequently to review any changes additions or updates highlighted in the history tab above. | ||
Discussion and contributions are similarly welcomed in the discussion tab above.[https://thehillside.info/index.php?title=Talk:Ventilation_and_COVID-19#section1] | |||
==Background== | ==Background== | ||
===Transmission routes=== | ===Transmission routes=== | ||
SARS-CoV-2 | SARS-CoV-2 has caused many to revisit their understanding of droplet and airborne transmission. These two transmission mechanisms form a continuum, but the following is generally accepted: | ||
*''Infectious'' particles <5μm in size can remain suspended and viable for many hours and these contribute to the risk of '''airborne transmission'''. | *''Infectious'' particles <5μm in size can remain suspended and viable for many hours and these contribute to the risk of '''airborne transmission'''. | ||
*'''Droplet transmission''' involves larger particles which can also spread through the air for some distance, but the range of transmission is generally considered to be less than 2 meters where after particles fall out of the breathing zone. It is important to remember that within this 2 m distance these larger droplets are essentially 'airborne' and diluting ventilation systems have little effect on reducing the risk of near-range droplet transmission<ref>Liu, L., Li, Y., Nielsen, P. V., Wei, J. & Jensen, R. L. Short-range airborne transmission of expiratory droplets between two people. Indoor Air 1–11 (2016) doi:10.1111/ina.12314.</ref>. | *'''Droplet transmission''' involves larger particles which can also spread through the air for some distance, but the range of transmission is generally considered to be less than 2 meters where after particles fall out of the breathing zone. It is important to remember that within this 2 m distance these larger droplets are essentially 'airborne' and diluting ventilation systems have little effect on reducing the risk of near-range droplet transmission<ref>Liu, L., Li, Y., Nielsen, P. V., Wei, J. & Jensen, R. L. Short-range airborne transmission of expiratory droplets between two people. Indoor Air 1–11 (2016) doi:10.1111/ina.12314.</ref>. | ||
Droplet precautions, therefore, include standard precautions like PPE, hand washing and distancing while airborne precautions include negative pressure isolation, respiratory protection, special exhaust or filtration regimes, etc. | Droplet precautions, therefore, include standard precautions like PPE, hand washing and distancing, while airborne precautions include negative pressure isolation, respiratory protection, special exhaust or filtration regimes, etc. | ||
Diseases seldom obey only one mode of transmission (obligatory transmission) but often have preferences (preferential transmission) while occasionally exploiting circumstances which provide rare opportunities for transmission (opportunistic routes). SARS-COV-2 is understood to be '''preferentially droplet | Diseases seldom obey only one mode of transmission (obligatory transmission) but often have preferences (preferential transmission) while occasionally exploiting circumstances which provide rare opportunities for transmission (opportunistic routes). SARS-COV-2 is understood to be '''preferentially droplet spread''' with opportunistic airborne spread possible in specific conditions, although an extensive outbreak review revealed no indication of airborne spread as defined for TB or measles<ref>https://www.who.int/news-room/commentaries/detail/modes-of-transmission-of-virus-causing-covid-19-implications-for-ipc-precaution-recommendations</ref>. | ||
===Airborne Transmission=== | ===Airborne Transmission=== | ||
There is still little strong evidence of common long-range airborne transmission in the sense of droplet nucleation, as with TB and measles<ref>World Health Organization. Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19) 16-24 February 2020 [Internet]. Geneva: World Health Organization; 2020 Available from: [https://www.who.int/docs/default- source/coronaviruse/who-china-joint-mission-on-covid-19-final-report.pdf https://www.who.int/docs/default- source/coronaviruse/who-china-joint-mission-on-covid-19-final-report.pdf]</ref>. Where evidence of airborne transmission has been reported, this can be seen in the context of opportunistic long-range droplet spread<ref> Wenzhao Chen, Nan Zhang, Jianjian Wei, Hui-LingYen, and Yuguo Li, “Short-range airborne route dominates exposure of respiratory infection during close contact,” medRxiv preprint, https://doi.org/10.1101/2020.03.16.20037291</ref>. A discussion contextualizing the reported cases of airborne transmission is discussed below. | There is still little strong evidence of common long-range airborne transmission in the sense of droplet nucleation, as with TB and measles<ref>World Health Organization. Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19) 16-24 February 2020 [Internet]. Geneva: World Health Organization; 2020 Available from: [https://www.who.int/docs/default- source/coronaviruse/who-china-joint-mission-on-covid-19-final-report.pdf https://www.who.int/docs/default- source/coronaviruse/who-china-joint-mission-on-covid-19-final-report.pdf]</ref>. Where evidence of airborne transmission has been reported, this can be seen in the context of opportunistic long-range droplet spread<ref> Wenzhao Chen, Nan Zhang, Jianjian Wei, Hui-LingYen, and Yuguo Li, “Short-range airborne route dominates exposure of respiratory infection during close contact,” medRxiv preprint, https://doi.org/10.1101/2020.03.16.20037291</ref>. A discussion contextualizing the reported cases of airborne transmission is discussed below. | ||
====van Doremalen et al (NEMJ 2020)==== | |||
The van Doremalen SARS-CoV-2 survival study<ref name="van Doremalen">Neeltje van Doremalen, Trenton Bushmaker, Dylan H. Morris, Myndi G. Holbrook, Amandine Gamble, Brandi N. Williamson, Azaibi Tamin, Jennifer L. Harcourt, Natalie J. Thornburg, Susan I. Gerber, James O. LloydSmith, Emmie de Wit, and Vincent J. Munster, “Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1,” The New England Journal of Medicine (2020), DOI: 10.1056/NEJMc2004973 [https://www.nejm.org/doi/pdf/10.1056/NEJMc2004973?articleTools=true]</ref> is often incorrectly reported to have shown that SARS-CoV-2 can remain viable in air for extended periods. No evidence for long range airborne viability has yet been found outside of lab settings. SARS-CoV-2 virus found dispersed at long range has not been cultured to prove viability and many studies have failed to detect it directly in air in quantities substantial enough to culture<ref>Faridi, S. et al. A field indoor air measurement of SARS-CoV-2 in the patient rooms of the largest hospital in Iran. Sci. Total Environ. 725, 1–5 (2020).</ref><ref>Liu, Y. et al. Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitals. Nature (2020) doi:10.1038/s41586-020-2271-3.</ref>. Correlations between culture viability, particle size and the real world infectious quantum were not described in this study<ref name="van Doremalen" /> as it was not the study's intention to claim COVID-19 was airborne. A more recent pre-publication article has made similar findings<ref>Fears SC, Klimstra WB, Duprex P, Hartman A, Weaver SC, Plante KS, et al. Persistence of severe acute respiratory syndrome coronavirus 2 in aerosol suspensions. Emerg Infect Dis. 2020 Sep [''date cited'']. <nowiki>https://doi.org/10.3201/eid2609.201806</nowiki></ref> but this has significant problems with equipment standardization and repeatability. More importantly, similar lab studies have also demonstrated a 3h airborne survival for viral strains such as Ebola not considered to be airborne<ref>Robert Comparison of the Aerosol Stability of 2 Strains of Zaire ebolavirus From the 1976 and 2013 Outbreaks Robert J. Fischer, Trenton Bushmaker, Seth Judson, Vincent J. Munster | The van Doremalen SARS-CoV-2 survival study<ref name="van Doremalen">Neeltje van Doremalen, Trenton Bushmaker, Dylan H. Morris, Myndi G. Holbrook, Amandine Gamble, Brandi N. Williamson, Azaibi Tamin, Jennifer L. Harcourt, Natalie J. Thornburg, Susan I. Gerber, James O. LloydSmith, Emmie de Wit, and Vincent J. Munster, “Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1,” The New England Journal of Medicine (2020), DOI: 10.1056/NEJMc2004973 [https://www.nejm.org/doi/pdf/10.1056/NEJMc2004973?articleTools=true]</ref> is often incorrectly reported to have shown that SARS-CoV-2 can remain viable in air for extended periods. No evidence for long range airborne viability has yet been found outside of lab settings. SARS-CoV-2 virus found dispersed at long range has not been cultured to prove viability and many studies have failed to detect it directly in air in quantities substantial enough to culture<ref>Faridi, S. et al. A field indoor air measurement of SARS-CoV-2 in the patient rooms of the largest hospital in Iran. Sci. Total Environ. 725, 1–5 (2020).</ref><ref>Liu, Y. et al. Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitals. Nature (2020) doi:10.1038/s41586-020-2271-3.</ref>. Correlations between culture viability, particle size and the real world infectious quantum were not described in this study<ref name="van Doremalen" /> as it was not the study's intention to claim COVID-19 was airborne. A more recent pre-publication article has made similar findings<ref>Fears SC, Klimstra WB, Duprex P, Hartman A, Weaver SC, Plante KS, et al. Persistence of severe acute respiratory syndrome coronavirus 2 in aerosol suspensions. Emerg Infect Dis. 2020 Sep [''date cited'']. <nowiki>https://doi.org/10.3201/eid2609.201806</nowiki></ref> but this has significant problems with equipment standardization and repeatability. More importantly, similar lab studies have also demonstrated a 3h airborne survival for viral strains such as Ebola not considered to be airborne<ref>Robert Comparison of the Aerosol Stability of 2 Strains of Zaire ebolavirus From the 1976 and 2013 Outbreaks Robert J. Fischer, Trenton Bushmaker, Seth Judson, Vincent J. Munster | ||
J Infect Dis. 2016 Oct 15; 214(Suppl 3): S290–S293. Published online 2016 Oct 4. doi: 10.1093/infdis/jiw193 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5050463/</ref>. This makes the direct application of this lab study in real-world settings problematic. | J Infect Dis. 2016 Oct 15; 214(Suppl 3): S290–S293. Published online 2016 Oct 4. doi: 10.1093/infdis/jiw193 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5050463/</ref>. This makes the direct application of this lab study in real-world settings problematic. Therefore, the understanding of the mechanisms of COVID-19 transmission is still largely reliant on what is understood of SARS (SARS-CoV-1)<ref>Isao Arita, Kazunobu Kojima, and Miyuki Nakane, “Transmission of severe acute respiratory syndrome,” Emerging. Infectious Diseases 9 No. 9 (2003):1183-84, [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3016764/].</ref>. | ||
====Guangzhou Restaurant Outbreak (2020)==== | |||
[[File:Guangzhou Restaurant COVID-19 2020.png|thumb|Plan of COVID-19 outbreak in Guangzhou Restaurant 2020<ref name=":0">Lu, J., Gu, J., Li, K., Xu, C., Su, W., Lai, Z....Yang, Z. (2020). COVID-19 Outbreak Associated with Air Conditioning in Restaurant, Guangzhou, China, 2020. ''Emerging Infectious Diseases'', ''26''(7), 1628-1631. <nowiki>https://dx.doi.org/10.3201/eid2607.200764</nowiki>.[https://wwwnc.cdc.gov/eid/article/26/7/20-0764_article]</ref>]] | |||
The 2020 outbreak of COVID-19 in a restaurant in Guangzhou<ref name=":0" /> raises some important questions around the airborne spread of the disease. This study shows that the transmission range of COVID-19 may exceed the generally prescribed separation distance of 1m under certain conditions, but fails to do so convincingly. Confounding issues that are not addressed adequately in the articles conclusion include: | |||
*the high probability of asymptomatic or pre-symptomatic spread of the virus from members of the index case's family<ref>How Coronavirus Infected Some, but Not All, in a Restaurant, Chang, K (2020) https://www.nytimes.com/2020/04/20/health/airflow-coronavirus-restaurants.html</ref> | |||
*the possibility of onward transmission within family groups after the restaurant exposure is acknowledged but dismissed without discussion | |||
*the difference in exposure times<ref>https://english.elpais.com/spanish_news/2020-06-17/an-analysis-of-three-covid-19-outbreaks-how-they-happened-and-how-they-can-be-avoided.html</ref> between tables (C-B )and (E-F) is not adequately addressed | |||
*The overcrowded and under ventilated conditions in the restaurant. | |||
This is a seminal event in the study of SARS-CoV-2 transmission, but we should be cautious to use it a clear evidence if airborne transmission where similar events are not widespread by now. | |||
===='''South Korea Call Centre Outbreak 2020'''==== | |||
[[File:South Korea Call Centre Outbreak COVID-19 2020.png|thumb|Floor plan of South Korea Call Centre Outbreak COVID-19 2020]] | |||
In this pre-publication report, the outbreak in a call-centre on the 11th story of a South Korean office block<ref>Park SY, Kim YM, Yi S, Lee S, Na BJ, Kim CB, et al. Coronavirus disease outbreak in call center, South Korea. Emerg Infect Dis. 2020 Aug [''date cited'']. <nowiki>https://doi.org/10.3201/eid2608.201274</nowiki></ref> offer some extraordinary insights but leaves many questions open. The distribution of the attacks is alarming in the call centre room, but is significantly reduced in adjacent room on the same floor. | |||
The following is a summary of the findings: | |||
*It appears as if the outbreak followed physical compartmentalization and not HVAC zoning although an HVAC plan of the building was not discussed. | |||
*It is clear that COVID-19 is exceptionally contagious in crowded office settings. | |||
*Lobbies and lifts contributed little to spread. | |||
*Exposure time correlated with transmission risk. | |||
Questions that remain: | |||
< | *HVAC zoning or an HVAC plan of the building was not discussed. | ||
*Ratios of male and female cases would have offered insight into the roles of bathrooms in COVID-19 spread. | |||
*A review of vertical transport characteristics may have offered insight into the vertical distribution of case through the building. | |||
===Aircraft Transmission Studies=== | |||
SARS and COVID-19 outbreaks on commercial aircraft have proven to be remarkedly rare. This may be due to the high ventilation rates<ref>Mangili, A., & Gendreau, M. A. (2005). Transmission of infectious diseases during commercial air travel. ''Lancet (London, England)'', ''365''(9463), 989–996. <nowiki>https://doi.org/10.1016/S0140-6736(05)71089-8</nowiki></ref>. Studies tracing contacts on flights seem to show multiple cases of very low to zero transmission rates with the transmission events raising disproportional alarm<ref name=":3">Olsen et al, N Engl J Med 2003; 349:2416-2422Transmission of the Severe Acute Respiratory Syndrome on Aircraft, DOI: 10.1056/NEJMoa031349 [https://www.nejm.org/doi/full/10.1056/nejmoa031349]</ref><ref>CMAJ 2020 April 14;192:E410. doi: 10.1503/cmaj.75015 [https://www.cmaj.ca/content/cmaj/192/15/E410.full.pdf]</ref>. The context of the scope of aircraft outbreak findings highlights the role ventilation has in creating safe environments, but similarly reveals the low risk levels associated with airborne transmission of SARS or COVID-19. | |||
====Amoy Gardens SARS Outbreak (2003)<ref name=":1">McKinney KR, Gong YY, Lewis TG. Environmental transmission of SARS at Amoy Gardens. ''J Environ Health''. 2006;68(9):26-52.</ref>==== | |||
Studies, which indicate the Amoy Gardens building's SARS outbreaks' transmission was via the airborne route<ref name=":1" />, commonly reference the prevailing wind between buildings. It should be noted that, since these buildings are about 60m apart, the environmental dilution and concentration decay effects are so strong it is not feasible that an infectious dose persists at that range. Similarly, the possibility that air can commute out of one window and into another needs to account for these dilution effects before assumptions of transmission can be drawn. These studies do not sufficiently account for dilution, infectious doses and pathogen survival rates. A more feasible hypothesis is that the Amoy Gardens intra-building spread was through re-aerosolisation of contaminated waste water coming from the faulty plumbing system. Similar outbreaks have more recently been found<ref name=":2">Bhowmick, G.D., Dhar, D., Nath, D. et al. Coronavirus disease 2019 (COVID-19) outbreak: some serious consequences with urban and rural water cycle. npj Clean Water 3, 32 (2020). https://doi.org/10.1038/s41545-020-0079-1</ref>. The re-aerosolisation from sanitary systems should be directly compared with long range oral-airborne transmission. The Amoy-Gardens transmission mode was most likely closer to large droplet transmission from toilets and waste outlets. | |||
====Other studies==== | |||
Studies which have found real-world SARS-CoV-2 in air, ducting and on extraction fans have so far failed to prove that the virus found was still viable<ref>Santarpia et al, “Transmission Potential of SARS-CoV-2 in Viral Shedding Observed at the University of Nebraska Medical Center,. medRxiv preprint (2020), [https://doi.org/10.1101/2020.03.23.20039446]</ref><ref>Po Ying Chia et al, 2020 (Preprint) “Detection of Air and Surface Contamination by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) in Hospital Rooms of Infective Patients,” medRxiv preprint (2020), https://doi.org/10.1101/2020.03.29.20046557 [https://www.medrxiv.org/content/10.1101/2020.03.29.20046557v2.full.pdf]</ref>. Air sampling studies have failed to detect viable SARS-CoV-2<ref>Ong SWX, Tan YK, Chia PY, et al. Air, Surface Environmental, and Personal Protective Equipment Contamination by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) From a Symptomatic Patient. ''JAMA.'' 2020;323(16):1610–1612. doi:10.1001/jama.2020.3227</ref>. | |||
It has been suggested that high temperature and humidity would reduce the spread of the virus<ref>Chin, A. W. H. et al. Stability of SARS-CoV-2 in different environmental conditions. The Lancet Microbe 0–4 (2020) doi:10.1016/s2666-5247(20)30003-3.</ref><ref>Pyankov, O. V., Bodnev, S. A., Pyankova, O. G. & Agranovski, I. E. Survival of aerosolized coronavirus in the ambient air. J. Aerosol Sci. 115, (2018).</ref>. The temperature ranges suggested (>50°C) are beyond what anyone could endure in an ICU but the humidity ranges of between 40-60% are achievable. The high humidity slows the nucleation of the viral droplet and increases its settling speed, thereby reducing its range. | |||
====High Risk Settings (ICU)==== | ====High Risk Settings (ICU)==== | ||
Much of the work being done to understand the transmission mechanism of COVID-19 is focused on community transmission. It is important to remember that transmission risk in an ICU will not be the same as in homes and workplaces. The conditions and procedures in ICUs could promote transmission - see WHO 2020 below<ref name="WHO 2020">WHO 2020, Modes of transmission of virus causing COVID-19: implications for IPC precaution recommendations https://www.who.int/news-room/commentaries/detail/modes-of-transmission-of-virus-causing-covid-19-implications-for-ipc-precaution-recommendations</ref>. Firstly, in a COVID ICU unit, the contamination source strength is much higher than other spaces since infected patients are congregated there. These are presumably ill patients with high viral shedding. Secondly, procedures like intubation are understood to release high quantities of aerosolized particles, unlike with general talking or coughing. Additionally, viral shedding through talking and coughing can be more readily mitigated than from intubation. | Much of the work being done to understand the transmission mechanism of COVID-19 is focused on community transmission. It is important to remember that transmission risk in an ICU will not be the same as in homes and workplaces. The conditions and procedures in ICUs could promote transmission - see WHO 2020 below<ref name="WHO 2020">WHO 2020, Modes of transmission of virus causing COVID-19: implications for IPC precaution recommendations https://www.who.int/news-room/commentaries/detail/modes-of-transmission-of-virus-causing-covid-19-implications-for-ipc-precaution-recommendations</ref>. Firstly, in a COVID ICU unit, the contamination source strength is much higher than other spaces since infected patients are congregated there. These are presumably ill patients with high viral shedding. Secondly, procedures like intubation are understood to release high quantities of aerosolized particles, unlike with general talking or coughing. Additionally, viral shedding through talking and coughing can be more readily mitigated than from intubation. | ||
===Fecal-Oral Transmission=== | ===Fecal-Oral Transmission=== | ||
Fecal oral route of transmission is acknowledged for COVID-19<ref>Pan Y, Zhang D, Yang P, Poon LLM, Wang Q. Viral load of SARS-CoV-2 in clinical samples. Lancet Infect Dis. 2020;20(4):411-2.</ref> and considerations for waste water management are discussed [[SARS-CoV-2 is found in faecal matter|here]] and [https://doi.org/10.1016/j.scitotenv.2020.139076 here]<ref>Kitajima et al,SARS-CoV-2 in wastewater: State of the knowledge and research needs,Science of The Total Environment,Volume 739,2020,139076,ISSN 0048-9697,<nowiki>https://doi.org/10.1016/j.scitotenv.2020.139076</nowiki></ref> | Fecal oral route of transmission is acknowledged for COVID-19<ref>Pan Y, Zhang D, Yang P, Poon LLM, Wang Q. Viral load of SARS-CoV-2 in clinical samples. Lancet Infect Dis. 2020;20(4):411-2.</ref> and considerations for waste water management are discussed [[SARS-CoV-2 is found in faecal matter|here]] and [https://doi.org/10.1016/j.scitotenv.2020.139076 here]<ref>Kitajima et al,SARS-CoV-2 in wastewater: State of the knowledge and research needs,Science of The Total Environment,Volume 739,2020,139076,ISSN 0048-9697,<nowiki>https://doi.org/10.1016/j.scitotenv.2020.139076</nowiki></ref>. This transmission route indirectly affects ventilation system design as special consideration should be given to common scenarios where the aerosolisation of contaminated wastewater is a possibility such as in bathrooms, sluice rooms and slurry pumping. These spaces should be well-ventilated and kept under negative pressure relative to adjacent spaces. | ||
This transmission route indirectly affects ventilation system design as special consideration should be given to common scenarios where the aerosolisation of contaminated wastewater is a possibility such as in bathrooms, sluice rooms and slurry pumping. These spaces should be well-ventilated and kept under negative pressure relative to adjacent spaces. | |||
==Institutional Guidance== | ==Institutional Guidance== | ||
| Line 55: | Line 85: | ||
"Airborne Infection Isolation Rooms (AIIRs) (See definition of AIIR in appendix) should be reserved for patients who will be undergoing aerosol generating procedures (See Aerosol Generating Procedures Section)."<ref>Interim Infection Prevention and Control Recommendations for Healthcare Personnel During the Coronavirus Disease 2019 (COVID-19) Pandemic (updated July 9, 2020)[https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control-recommendations.html]</ref> | "Airborne Infection Isolation Rooms (AIIRs) (See definition of AIIR in appendix) should be reserved for patients who will be undergoing aerosol generating procedures (See Aerosol Generating Procedures Section)."<ref>Interim Infection Prevention and Control Recommendations for Healthcare Personnel During the Coronavirus Disease 2019 (COVID-19) Pandemic (updated July 9, 2020)[https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control-recommendations.html]</ref> | ||
This nuanced approach is difficult to tease out of the guidance from engineering societies. | This nuanced approach is difficult to tease out of the guidance from engineering societies. | ||
{{Anchor|CDC COVID Airborne Precautions}} | |||
====Airborne Transmission based precautions (US-CDC)==== | |||
The CDC has made the following recommendations for transmission based precautions. It is notable that this is only for "non-US settings". | |||
Additionally, adequately ventilated single rooms or wards are suggested. For general ward rooms with natural ventilation, adequate ventilation for COVID-19 patients is considered to be 60 L/s per patient. When single rooms are not available, suspected COVID-19 patients should be grouped together with beds at least 1 meter apart based on WHO’s recommendation, although some member states have recommended maintaining greater distances whenever possible<ref name=":4">CDC 2019, COVID-19 Overview and Infection Prevention and Control Priorities in Non-US Healthcare Settings https://www.cdc.gov/coronavirus/2019-ncov/hcp/non-us-settings/overview/index.html </ref> | |||
This guidance offers no reason or evidence for the suggestion of 60 L/s per patient. It may stem from the WHO's recommendations for 60 L/s per person for medium risk TB settings<ref>WHO 2009. WHO Policy on TB infection control in Health-care facilities, Congregate Settings and Households. [https://www.who.int/tb/publications/tb-facilities-policy/en/ https://apps.who.int/iris/bitstream/handle/10665/44148/9789241598323_eng.pdf?sequence=1]</ref> as it contradicts the CDC's own TB guidance <ref>CDC 2005, Guidelines for Environmental Infection Control in Health-Care Facilities (updated 2019)https://www.cdc.gov/mmwr/pdf/rr/rr5417.pdf</ref>. It is of significance to note that the WHO has omitted this recommendation from its 2019 guidance <ref>WHO 2019, WHO Guidelines on tuberculosis infection prevention and control, 2019 update https://www.who.int/tb/publications/2019/guidelines-tuberculosis-infection-prevention-2019/en/</ref>. This guidance is not prescriptive, supported by evidence nor consistent with other CDC and WHO guidance. Additionally the guidance does not describe how the ventilation performance for naturally ventilated spaces should be verified. See [[#COVID-19 Engineering Response]] for recommended practice | |||
===ASHRAE=== | ===ASHRAE=== | ||
While the US-CDC and WHO maintains that the airborne transmission is possible but not common or of primary concern, ASHRAE (being an association dedicated to ventilation engineering) focuses on the airborne component. | While the US-CDC and WHO maintains that the airborne transmission is possible but not common or of primary concern, ASHRAE (being an association dedicated to ventilation engineering) focuses on the airborne component. | ||
"Transmission of SARS-CoV-2 through the air is sufficiently likely that airborne exposure to the virus should be controlled. Changes to building operations, including the operation of heating, ventilating, and air-conditioning systems, can reduce airborne exposures"<ref>Q: Does ASHRAE’s guidance agree with guidance from WHO and CDC?[https://www.ashrae.org/file%20library/technical%20resources/covid-19/does-ashrae-s-guidance-agree-with-guidance-from-who-and-cdc.pdf]</ref> | "Transmission of SARS-CoV-2 through the air is sufficiently likely that airborne exposure to the virus should be controlled. Changes to building operations, including the operation of heating, ventilating, and air-conditioning systems, can reduce airborne exposures"<ref>Q: Does ASHRAE’s guidance agree with guidance from WHO and CDC? (ASHRAE 2020)[https://www.ashrae.org/file%20library/technical%20resources/covid-19/does-ashrae-s-guidance-agree-with-guidance-from-who-and-cdc.pdf]</ref> | ||
ASHRAE makes useful distinctions between guidance for healthcare<ref>ASHRAE healthcare C19 guidance (ASHRAE 2020) [https://www.ashrae.org/file%20library/technical%20resources/covid-19/ashrae-healthcare-c19-guidance.pdf]</ref>, residential <ref>ASHRAE residential c19 guidance (ASHRAE 2020)[https://www.ashrae.org/file%20library/technical%20resources/covid-19/ashrae-residential-c19-guidance.pdf]</ref><ref>COVID 19 guidance for multifamily building owners-managers (ASHRAE 2020)[https://www.ashrae.org/file%20library/technical%20resources/covid-19/covid-19-guidance-for-multifamily-building-owners_managers.pdf]</ref>, commercial <ref>ASHRAE commercial C19 guidance (ASHRAE 2020)[https://www.ashrae.org/file%20library/technical%20resources/covid-19/ashrae-commercial-c19-guidance.pdf]</ref> and schools<ref>ASHRAE Schools C19 guidance (ASHRAE 2020)[https://www.ashrae.org/file%20library/technical%20resources/covid-19/ashrae-schools-c19-guidance.pdf</ref>, but doesn't significantly address risk categories specifically in healthcare or resource limited settings. | ASHRAE makes useful distinctions between guidance for healthcare<ref>ASHRAE healthcare C19 guidance (ASHRAE 2020) [https://www.ashrae.org/file%20library/technical%20resources/covid-19/ashrae-healthcare-c19-guidance.pdf]</ref>, residential <ref>ASHRAE residential c19 guidance (ASHRAE 2020)[https://www.ashrae.org/file%20library/technical%20resources/covid-19/ashrae-residential-c19-guidance.pdf]</ref><ref>COVID 19 guidance for multifamily building owners-managers (ASHRAE 2020)[https://www.ashrae.org/file%20library/technical%20resources/covid-19/covid-19-guidance-for-multifamily-building-owners_managers.pdf]</ref>, commercial <ref>ASHRAE commercial C19 guidance (ASHRAE 2020)[https://www.ashrae.org/file%20library/technical%20resources/covid-19/ashrae-commercial-c19-guidance.pdf]</ref> and schools<ref>ASHRAE Schools C19 guidance (ASHRAE 2020)[https://www.ashrae.org/file%20library/technical%20resources/covid-19/ashrae-schools-c19-guidance.pdf</ref>, but doesn't significantly address risk categories specifically in healthcare or resource limited settings. | ||
===REHVA=== | ===REHVA=== | ||
REHVA's temporary guidance is limited to commercial and public buildings<ref>REHVA COVID-19 guidance document, April 3, 2020[https://www.rehva.eu/fileadmin/user_upload/REHVA_COVID-19_guidance_document_ver2_20200403_1.pdf]</ref> | REHVA's temporary guidance is limited to commercial and public buildings<ref>REHVA COVID-19 guidance document, April 3, 2020[https://www.rehva.eu/fileadmin/user_upload/REHVA_COVID-19_guidance_document_ver2_20200403_1.pdf]</ref>. Similar to ASHRAE, REHVA focusses on engineering controls for airborne transmission. REHVA acknowledges importance of droplet precautions and the lack of quality evidence for airborne transmission, but draws the conclusion that SARS-CoV-2 RNA found in ventilation ducting implies airborne transmission, even though these real world studies have not yet proven viability of these particles. REHVA also draws the airborne conclusion from the van Doremalen study<ref name="van Doremalen" /> out of its intended comparative context. | ||
=== IUSS (2014) === | ===IUSS (2014)=== | ||
The IUSS Building Engineering Services Guidelines <ref>Building Engineering Services (2014)[https://iussonline.co.za/norms-standards/healthcare-environment/60-building-engineering-services]</ref>, which is mandated for new buildings by provincial departments of health by reference in Government Notice R116<ref>Government Notice R116 (17 Feb 2014)[https://iussonline.co.za/docman/gazettes/116-notice-37348/file]</ref>, describes risk based ventilation criteria which are broadly appropriate for COVID-19, if not excessive. This guideline was developed with control measures for the current TB epidemic in mind. These measures would be more than appropriate for most healthcare spaces. The guidance recommends no recirculation of air between theatres and adjacent spaces but does not prohibit cascading from surgeries to adjacent spaces. Therefore, confirmed COVID-19 patients should only be treated in negative pressure operating rooms that comply with the guidelines. | The [[Infrastructure_Unit_System_Support|IUSS]] Building Engineering Services Guidelines<ref>Building Engineering Services (2014)[https://iussonline.co.za/norms-standards/healthcare-environment/60-building-engineering-services]</ref>, which is mandated for new buildings by provincial departments of health by reference in Government Notice R116<ref>Government Notice R116 (17 Feb 2014)[https://iussonline.co.za/docman/gazettes/116-notice-37348/file]</ref>, describes risk based ventilation criteria which are broadly appropriate for COVID-19, if not excessive. This guideline was developed with control measures for the current TB epidemic in mind. These measures would be more than appropriate for most healthcare spaces. The guidance recommends no recirculation of air between theatres and adjacent spaces but does not prohibit cascading from surgeries to adjacent spaces. Therefore, confirmed COVID-19 patients should only be treated in negative pressure operating rooms that comply with the guidelines. | ||
== | ===SANS 10400-O (2011)=== | ||
The 2011 edition of the SANS 10400-O<ref>SABS. SANS 10400-O : 2011 SOUTH AFRICAN NATIONAL STANDARD The application of the National Building Regulations Part O : Lighting and Ventilation. (2011).</ref> is often criticized for over prescribing ventilation rates when compared with international best practice. The mechanical ventilation criteria of this standard, in it current form, prioritizes indoor air quality over energy efficiency and takes a heavy handed approach to ventilation in many spaces. 10 ACH and more is common for areas with any risk of airborne contamination. The standard gives inadequate performance guidance for naturally ventilated spaces. | |||
While the SANS 10400-O:2011 demands unprecedently high ventilation rates and offers little supporting evidence with the criteria, it should be considered better than many international standards for use in general settings where airborne contamination is a risk. | |||
Unfortunately, the SANS 10400-O:2011 does not permit the concurrent use of natural ventilation and air-conditioning and leads designers to incorrectly infer that windows in airconditioned spaces should not be opened/openable. | |||
==Air-Conditioning, Ventilation and COVID-19== | |||
It is important to differentiate between ventilation and air-conditioning when discussion indoor contamination. When the term ventilation is used, it describes any system that induces decontaminated, fresh or outdoor-air to enter a space by the application of supply or extraction systems. Diluting ventilation is the most commonly used regime. Other modes of contaminant removal include displacement and local exhaust ventilation systems, each of which requires its own nuanced discussion as they pertain to infection control. | |||
Air-conditioning in contrast, refers to only the mechanical cooling or heating system, sometimes installed directly in a space (Spit-AC), to offer thermal comfort and sometimes humidity control. In-room air-conditioning systems that circulate air directly within a space with no dilution or extraction can directly offer no reduction in airborne contaminant levels. In some instances they can even assist in the distribution of contaminants. | |||
Openable windows can be considered as ventilation apertures and, in most cases, offer highly effective ventilation. Unfortunately, this is sometimes at the expense of indoor comfort. Even though long range droplet transmission of SARS-CoV-2 is relatively low in comparison to short range transmission, encouraging occupants to open windows will reduce that risk. Allowing occupants to use air-conditioning to either heat or cool a space while windows are open can improve levels of open window compliance which is more important than limiting AC use for reducing long range transmission. An additional strategy to both improve open window compliance and reduce AC usage would be to relax strict corporate dress codes as this can improve thermal comfort levels seasonally. | |||
{{Anchor|COVID-19 Engineering Response}} | |||
==Engineering Response== | |||
Ventilation society guidance understandably bears the risk of being biased toward over-prescribing solutions over which engineers have the greatest understanding and control. It is within this context that the valuable guidance published online by REHVA and ASHRAE should be considered. Revamping existing ventilation systems in resource-constrained healthcare settings to meet admittedly overly-cautious guidance should not be conducted without an informed investment case. | Ventilation society guidance understandably bears the risk of being biased toward over-prescribing solutions over which engineers have the greatest understanding and control. It is within this context that the valuable guidance published online by REHVA and ASHRAE should be considered. Revamping existing ventilation systems in resource-constrained healthcare settings to meet admittedly overly-cautious guidance should not be conducted without an informed investment case. | ||
In these resource limited settings, it needs to be carefully considered whether resources are allocated to clinical capacity or to possibly unnecessary ventilation when the benefits of these criteria may be comparatively marginal. | |||
Without good viability studies of the viral particles found in ventilation systems, no firm guidance can be offered regarding the rate of reduction for SARS-CoV-2 viability with time and distance. Until that time it would be prudent to assume that the virus should only be considered as airborne under special and rare conditions, based on the guidance of the WHO. This would determine that we have different filtration and ventilation approaches between COVID-ICUs, general indoor public spaces and spaces with a potential for high density occupation. | Without good viability studies of the viral particles found in air or ventilation systems, no firm guidance can be offered regarding the rate of reduction for SARS-CoV-2 viability with time and distance. Until that time it would be prudent to assume that the virus should only be considered as airborne under special and rare conditions, based on the guidance of the WHO, and these conditions should be avoided. This would determine that we have different filtration and ventilation approaches between COVID-ICUs, general indoor public spaces and spaces with a potential for high density occupation. Engineers should not be tempted to assume or argue that all indoor spaces bear the same risk profile. | ||
For high-risk spaces it may be prudent to implement temporary measures to limit transmission risk to the minimum possible. In order of priorities, engineering interventions include: | For high-risk spaces it may be prudent to implement temporary measures to limit transmission risk to the minimum possible. In order of priorities, engineering interventions include: | ||
| Line 193: | Line 244: | ||
|Moderate | |Moderate | ||
|} | |} | ||
Therefore, assuming systems have been designed in accordance with the IUSS guidance, there should be little reason to change their configuration or pressurization unless general areas are repurposed as airborne precaution rooms. Risk assessments should be conducted for ICUs and COVID-19 wards immediately adjacent to public waiting areas or other high traffic areas, with corrective actions including but not limited to reducing occupancy times and rates for these areas. | |||
===Airborne precautions for settings with uncertain or high residual risk=== | |||
Where the verification of engineering responses are uncertain or the residual risk remains high, the following approach is proposed. | |||
Modelling ventilation rates for limiting transmission risk in the context of the proposed quantum generation rate<ref>Noakes, C. J. & Sleigh, P. A. Mathematical models for assessing the role of airflow on the risk of airborne infection in hospital wards. J. R. Soc. Interface 6, S791–S800 (2009). https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2843948/</ref> for airborne COVID-19 <ref>Zhao, Dai, preprint 2020, Association of infected probability of COVID-19 with ventilation rates in confined spaces: a Wells-Riley equation based investigation https://doi.org/10.1101/2020.04.21.20072397</ref> supports the conclusion that the COVID-19 ventilation rates should be not less that 59.7 L/s per person for an 8 hour exposure in a 100 seater waiting room, which (coincidentally) matches the WHO's previous recommendations for medium risk spaces and the CDC's adoption of that value for COVID-19 ([[Ventilation and COVID-19#CDC COVID Airborne Precautions|see above]]). | |||
Reducing the exposure time would reduce the required ventilation rate. 8h is assumed to keep staff and patients equally "safe". Obviously additional precautions are recommended for health care staff to control for repeated daily exposure. | |||
A significant problem with the WHO and CDC recommendation of 60 L/s per patient (person) is that an individual's risk increases from 1% to nearly 40% as this criteria is applied to room populations decreasing from 100 persons. | |||
To maintain a personal risk below 1% the following correction is advised: | |||
<math>Q/n = 60 \times \bigl(A/n) </math> | |||
<math>\therefore Q = 60 \times \bigl(m^2 per person) </math> | |||
Where '''Q''' is the ventilation rate <math>(L/s\cdot m^2)</math>, '''A''' is the floor area <math>(m^2)</math> and n is the maximum number of people in the space.<br />Air changes per hour (ACH) can be estimated with <math>ACH = Q \times 3.6 \div h </math>, where <math>h</math> is the ceiling height. | |||
Assuming face masks reduce transmission by 40%, ventilation rates of '''38''' <math>L/s\cdot person</math> would be required to control airborne transmission. This sounds like a lot until the social distancing and decongestion measures required for droplet precautions are applied. under these conditions this equates to only '''4.23''' <math>L/s\cdot m^2</math> or between 4 and 5 ACH (assuming a 3.2m ceiling height). This should be easily achievable for naturally ventilated spaces and could be verified by measuring average indoor CO<sub>2</sub> concentrations no greater than '''170 PPM''' above outdoor. | |||
==Conclusion== | |||
Therefore, assuming ventilation systems in South Africa have been designed in accordance with the IUSS guidance, there should be little reason to change their configuration or pressurization unless general areas are repurposed as airborne precaution rooms. Risk assessments should be conducted for ICUs and COVID-19 wards immediately adjacent to public waiting areas or other high traffic areas, with corrective actions including but not limited to reducing occupancy times and rates for these areas and adjusting distancing rules. | |||
<br /> | <br /> | ||
| Line 202: | Line 271: | ||
[[Category:COVID-19]] | [[Category:COVID-19]] | ||
[[Category:ICU]] | [[Category:ICU]] | ||
<references /> | <references /> | ||
[[Category:ASHRAE]] | |||
[[Category:REHVA]] | |||
[[Category:IUSS]] | |||
[[Category:Airborne Infection control]] | |||
[[Category:Airborne Contamination Control]] | |||
Latest revision as of 14:56, 19 August 2021
Context
This article aims to to contextualize COVID-19 related ventilation guidelines in a field of developing clinical evidence. This is done with the hope of empowering the reader to scrutinize proposed interventions within this context and employ appropriate and efficient solutions. The information and guidance in this article is the developing opinion of the author and does not represent any regulatory or institutional mandate or authority. The evidence supporting this opinion is evolving and therefore the opinion is similarly subject to change. The reader is encouraged to return to this article frequently to review any changes additions or updates highlighted in the history tab above.
Discussion and contributions are similarly welcomed in the discussion tab above.[17]
Background
Transmission routes
SARS-CoV-2 has caused many to revisit their understanding of droplet and airborne transmission. These two transmission mechanisms form a continuum, but the following is generally accepted:
- Infectious particles <5μm in size can remain suspended and viable for many hours and these contribute to the risk of airborne transmission.
- Droplet transmission involves larger particles which can also spread through the air for some distance, but the range of transmission is generally considered to be less than 2 meters where after particles fall out of the breathing zone. It is important to remember that within this 2 m distance these larger droplets are essentially 'airborne' and diluting ventilation systems have little effect on reducing the risk of near-range droplet transmission[1].
Droplet precautions, therefore, include standard precautions like PPE, hand washing and distancing, while airborne precautions include negative pressure isolation, respiratory protection, special exhaust or filtration regimes, etc.
Diseases seldom obey only one mode of transmission (obligatory transmission) but often have preferences (preferential transmission) while occasionally exploiting circumstances which provide rare opportunities for transmission (opportunistic routes). SARS-COV-2 is understood to be preferentially droplet spread with opportunistic airborne spread possible in specific conditions, although an extensive outbreak review revealed no indication of airborne spread as defined for TB or measles[2].
Airborne Transmission
There is still little strong evidence of common long-range airborne transmission in the sense of droplet nucleation, as with TB and measles[3]. Where evidence of airborne transmission has been reported, this can be seen in the context of opportunistic long-range droplet spread[4]. A discussion contextualizing the reported cases of airborne transmission is discussed below.
van Doremalen et al (NEMJ 2020)
The van Doremalen SARS-CoV-2 survival study[5] is often incorrectly reported to have shown that SARS-CoV-2 can remain viable in air for extended periods. No evidence for long range airborne viability has yet been found outside of lab settings. SARS-CoV-2 virus found dispersed at long range has not been cultured to prove viability and many studies have failed to detect it directly in air in quantities substantial enough to culture[6][7]. Correlations between culture viability, particle size and the real world infectious quantum were not described in this study[5] as it was not the study's intention to claim COVID-19 was airborne. A more recent pre-publication article has made similar findings[8] but this has significant problems with equipment standardization and repeatability. More importantly, similar lab studies have also demonstrated a 3h airborne survival for viral strains such as Ebola not considered to be airborne[9]. This makes the direct application of this lab study in real-world settings problematic. Therefore, the understanding of the mechanisms of COVID-19 transmission is still largely reliant on what is understood of SARS (SARS-CoV-1)[10].
Guangzhou Restaurant Outbreak (2020)
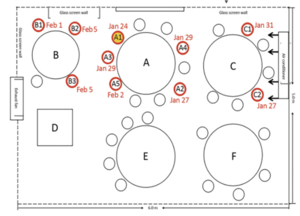
The 2020 outbreak of COVID-19 in a restaurant in Guangzhou[11] raises some important questions around the airborne spread of the disease. This study shows that the transmission range of COVID-19 may exceed the generally prescribed separation distance of 1m under certain conditions, but fails to do so convincingly. Confounding issues that are not addressed adequately in the articles conclusion include:
- the high probability of asymptomatic or pre-symptomatic spread of the virus from members of the index case's family[12]
- the possibility of onward transmission within family groups after the restaurant exposure is acknowledged but dismissed without discussion
- the difference in exposure times[13] between tables (C-B )and (E-F) is not adequately addressed
- The overcrowded and under ventilated conditions in the restaurant.
This is a seminal event in the study of SARS-CoV-2 transmission, but we should be cautious to use it a clear evidence if airborne transmission where similar events are not widespread by now.
South Korea Call Centre Outbreak 2020

In this pre-publication report, the outbreak in a call-centre on the 11th story of a South Korean office block[14] offer some extraordinary insights but leaves many questions open. The distribution of the attacks is alarming in the call centre room, but is significantly reduced in adjacent room on the same floor.
The following is a summary of the findings:
- It appears as if the outbreak followed physical compartmentalization and not HVAC zoning although an HVAC plan of the building was not discussed.
- It is clear that COVID-19 is exceptionally contagious in crowded office settings.
- Lobbies and lifts contributed little to spread.
- Exposure time correlated with transmission risk.
Questions that remain:
- HVAC zoning or an HVAC plan of the building was not discussed.
- Ratios of male and female cases would have offered insight into the roles of bathrooms in COVID-19 spread.
- A review of vertical transport characteristics may have offered insight into the vertical distribution of case through the building.
Aircraft Transmission Studies
SARS and COVID-19 outbreaks on commercial aircraft have proven to be remarkedly rare. This may be due to the high ventilation rates[15]. Studies tracing contacts on flights seem to show multiple cases of very low to zero transmission rates with the transmission events raising disproportional alarm[16][17]. The context of the scope of aircraft outbreak findings highlights the role ventilation has in creating safe environments, but similarly reveals the low risk levels associated with airborne transmission of SARS or COVID-19.
Amoy Gardens SARS Outbreak (2003)[18]
Studies, which indicate the Amoy Gardens building's SARS outbreaks' transmission was via the airborne route[18], commonly reference the prevailing wind between buildings. It should be noted that, since these buildings are about 60m apart, the environmental dilution and concentration decay effects are so strong it is not feasible that an infectious dose persists at that range. Similarly, the possibility that air can commute out of one window and into another needs to account for these dilution effects before assumptions of transmission can be drawn. These studies do not sufficiently account for dilution, infectious doses and pathogen survival rates. A more feasible hypothesis is that the Amoy Gardens intra-building spread was through re-aerosolisation of contaminated waste water coming from the faulty plumbing system. Similar outbreaks have more recently been found[19]. The re-aerosolisation from sanitary systems should be directly compared with long range oral-airborne transmission. The Amoy-Gardens transmission mode was most likely closer to large droplet transmission from toilets and waste outlets.
Other studies
Studies which have found real-world SARS-CoV-2 in air, ducting and on extraction fans have so far failed to prove that the virus found was still viable[20][21]. Air sampling studies have failed to detect viable SARS-CoV-2[22].
It has been suggested that high temperature and humidity would reduce the spread of the virus[23][24]. The temperature ranges suggested (>50°C) are beyond what anyone could endure in an ICU but the humidity ranges of between 40-60% are achievable. The high humidity slows the nucleation of the viral droplet and increases its settling speed, thereby reducing its range.
High Risk Settings (ICU)
Much of the work being done to understand the transmission mechanism of COVID-19 is focused on community transmission. It is important to remember that transmission risk in an ICU will not be the same as in homes and workplaces. The conditions and procedures in ICUs could promote transmission - see WHO 2020 below[25]. Firstly, in a COVID ICU unit, the contamination source strength is much higher than other spaces since infected patients are congregated there. These are presumably ill patients with high viral shedding. Secondly, procedures like intubation are understood to release high quantities of aerosolized particles, unlike with general talking or coughing. Additionally, viral shedding through talking and coughing can be more readily mitigated than from intubation.
Fecal-Oral Transmission
Fecal oral route of transmission is acknowledged for COVID-19[26] and considerations for waste water management are discussed here and here[27]. This transmission route indirectly affects ventilation system design as special consideration should be given to common scenarios where the aerosolisation of contaminated wastewater is a possibility such as in bathrooms, sluice rooms and slurry pumping. These spaces should be well-ventilated and kept under negative pressure relative to adjacent spaces.
Institutional Guidance
WHO
The WHO's advice regarding SARS-CoV-2 transmission during clinical interventions is as follows:
"In the context of COVID-19, airborne transmission may be possible in specific circumstances and settings in which procedures or support treatments that generate aerosols are performed; i.e., endotracheal intubation, bronchoscopy, open suctioning, administration of nebulized treatment, manual ventilation before intubation, turning the patient to the prone position, disconnecting the patient from the ventilator, non-invasive positive-pressure ventilation, tracheostomy, and cardiopulmonary resuscitation." - WHO 2020[25]
While the WHO's position acknowledges the increased risk of transmission in overcrowded and under-ventilated spaces, the appropriate response is not to increase prescribed general ventilation rates, but rather to avoid overcrowding and maintain ventilation systems correctly.
US-CDC
The CDC's advice regarding SARS-CoV-2 transmission is still nearly identical to its guidance for SARS-CoV-1:
"The primary transmission of COVID-19 is from person-to-person through respiratory droplets. These droplets are released when someone with COVID-19 sneezes or coughs. COVID-19 can also be spread when you are in close contact with someone who is sick (e.g., shaking hands or talking). A physical distance of at least 1 meter (3ft) between persons is suggested by the World Health Organization (WHO) to avoid infection, although some WHO member states have recommended maintaining greater distances whenever possible. Respiratory droplets can land on objects or surfaces around the person when they cough or talk, and people can then become infected with COVID-19 from touching these objects or surfaces and then touching their eyes, nose or mouth. Recent data suggests that there can be transmission of COVID-19 through droplets of those with mild symptoms or those who do not feel ill" [28][29]
The US-CDC's recommendations regarding inpatient accommodation for SARS includes the comment,
"Experience in some settings in Taiwan and Toronto demonstrated that cohorting SARS patients, without use of AIIRs, effectively interrupted transmission"[30]
The CDC's guidance is consistent with the full context of hierarchical risk-based infection control and is suitably cognizant of variously resourced settings.
"Airborne Infection Isolation Rooms (AIIRs) (See definition of AIIR in appendix) should be reserved for patients who will be undergoing aerosol generating procedures (See Aerosol Generating Procedures Section)."[31]
This nuanced approach is difficult to tease out of the guidance from engineering societies.
Airborne Transmission based precautions (US-CDC)
The CDC has made the following recommendations for transmission based precautions. It is notable that this is only for "non-US settings".
Additionally, adequately ventilated single rooms or wards are suggested. For general ward rooms with natural ventilation, adequate ventilation for COVID-19 patients is considered to be 60 L/s per patient. When single rooms are not available, suspected COVID-19 patients should be grouped together with beds at least 1 meter apart based on WHO’s recommendation, although some member states have recommended maintaining greater distances whenever possible[32]
This guidance offers no reason or evidence for the suggestion of 60 L/s per patient. It may stem from the WHO's recommendations for 60 L/s per person for medium risk TB settings[33] as it contradicts the CDC's own TB guidance [34]. It is of significance to note that the WHO has omitted this recommendation from its 2019 guidance [35]. This guidance is not prescriptive, supported by evidence nor consistent with other CDC and WHO guidance. Additionally the guidance does not describe how the ventilation performance for naturally ventilated spaces should be verified. See #COVID-19 Engineering Response for recommended practice
ASHRAE
While the US-CDC and WHO maintains that the airborne transmission is possible but not common or of primary concern, ASHRAE (being an association dedicated to ventilation engineering) focuses on the airborne component.
"Transmission of SARS-CoV-2 through the air is sufficiently likely that airborne exposure to the virus should be controlled. Changes to building operations, including the operation of heating, ventilating, and air-conditioning systems, can reduce airborne exposures"[36]
ASHRAE makes useful distinctions between guidance for healthcare[37], residential [38][39], commercial [40] and schools[41], but doesn't significantly address risk categories specifically in healthcare or resource limited settings.
REHVA
REHVA's temporary guidance is limited to commercial and public buildings[42]. Similar to ASHRAE, REHVA focusses on engineering controls for airborne transmission. REHVA acknowledges importance of droplet precautions and the lack of quality evidence for airborne transmission, but draws the conclusion that SARS-CoV-2 RNA found in ventilation ducting implies airborne transmission, even though these real world studies have not yet proven viability of these particles. REHVA also draws the airborne conclusion from the van Doremalen study[5] out of its intended comparative context.
IUSS (2014)
The IUSS Building Engineering Services Guidelines[43], which is mandated for new buildings by provincial departments of health by reference in Government Notice R116[44], describes risk based ventilation criteria which are broadly appropriate for COVID-19, if not excessive. This guideline was developed with control measures for the current TB epidemic in mind. These measures would be more than appropriate for most healthcare spaces. The guidance recommends no recirculation of air between theatres and adjacent spaces but does not prohibit cascading from surgeries to adjacent spaces. Therefore, confirmed COVID-19 patients should only be treated in negative pressure operating rooms that comply with the guidelines.
SANS 10400-O (2011)
The 2011 edition of the SANS 10400-O[45] is often criticized for over prescribing ventilation rates when compared with international best practice. The mechanical ventilation criteria of this standard, in it current form, prioritizes indoor air quality over energy efficiency and takes a heavy handed approach to ventilation in many spaces. 10 ACH and more is common for areas with any risk of airborne contamination. The standard gives inadequate performance guidance for naturally ventilated spaces.
While the SANS 10400-O:2011 demands unprecedently high ventilation rates and offers little supporting evidence with the criteria, it should be considered better than many international standards for use in general settings where airborne contamination is a risk.
Unfortunately, the SANS 10400-O:2011 does not permit the concurrent use of natural ventilation and air-conditioning and leads designers to incorrectly infer that windows in airconditioned spaces should not be opened/openable.
Air-Conditioning, Ventilation and COVID-19
It is important to differentiate between ventilation and air-conditioning when discussion indoor contamination. When the term ventilation is used, it describes any system that induces decontaminated, fresh or outdoor-air to enter a space by the application of supply or extraction systems. Diluting ventilation is the most commonly used regime. Other modes of contaminant removal include displacement and local exhaust ventilation systems, each of which requires its own nuanced discussion as they pertain to infection control.
Air-conditioning in contrast, refers to only the mechanical cooling or heating system, sometimes installed directly in a space (Spit-AC), to offer thermal comfort and sometimes humidity control. In-room air-conditioning systems that circulate air directly within a space with no dilution or extraction can directly offer no reduction in airborne contaminant levels. In some instances they can even assist in the distribution of contaminants.
Openable windows can be considered as ventilation apertures and, in most cases, offer highly effective ventilation. Unfortunately, this is sometimes at the expense of indoor comfort. Even though long range droplet transmission of SARS-CoV-2 is relatively low in comparison to short range transmission, encouraging occupants to open windows will reduce that risk. Allowing occupants to use air-conditioning to either heat or cool a space while windows are open can improve levels of open window compliance which is more important than limiting AC use for reducing long range transmission. An additional strategy to both improve open window compliance and reduce AC usage would be to relax strict corporate dress codes as this can improve thermal comfort levels seasonally.
Engineering Response
Ventilation society guidance understandably bears the risk of being biased toward over-prescribing solutions over which engineers have the greatest understanding and control. It is within this context that the valuable guidance published online by REHVA and ASHRAE should be considered. Revamping existing ventilation systems in resource-constrained healthcare settings to meet admittedly overly-cautious guidance should not be conducted without an informed investment case.
In these resource limited settings, it needs to be carefully considered whether resources are allocated to clinical capacity or to possibly unnecessary ventilation when the benefits of these criteria may be comparatively marginal.
Without good viability studies of the viral particles found in air or ventilation systems, no firm guidance can be offered regarding the rate of reduction for SARS-CoV-2 viability with time and distance. Until that time it would be prudent to assume that the virus should only be considered as airborne under special and rare conditions, based on the guidance of the WHO, and these conditions should be avoided. This would determine that we have different filtration and ventilation approaches between COVID-ICUs, general indoor public spaces and spaces with a potential for high density occupation. Engineers should not be tempted to assume or argue that all indoor spaces bear the same risk profile.
For high-risk spaces it may be prudent to implement temporary measures to limit transmission risk to the minimum possible. In order of priorities, engineering interventions include:
- decongest indoor spaces to the minimum possible occupancy levels
- open windows to outside when occupational health, safety and security are not compromised
- increase HVAC fresh air rates to maximum possible levels
- reduce HVAC recirculation levels to minimum possible levels
- flush buildings with fresh air before and after daily occupancy
The following matrix is intended to guide our design responses for a sample of space types
| Space Type | Risk | Initial Risk | Engineering Response | Residual Risk |
|---|---|---|---|---|
| ICU | Transmission in ICU | Severe |
|
Low |
| Transmission to Adjacent spaces | Low |
|
Low | |
| Surgeries | Transmission in Theatre | Severe |
|
Low |
| Transmission to Adjacent spaces | Moderate |
|
Low | |
| COVID Wards | Transmission within COVID-19 Ward | Low |
|
Low |
| Transmission to Adjacent spaces | High |
|
Low | |
| General wards | Transmission within and from Ward | Low |
|
Low |
| Emergency centre | Transmission within EC | High |
|
Moderate |
| Hospital Waiting Areas | Transmission within waiting room | High |
|
Moderate |
| Other public waiting spaces | Transmission within waiting room | Moderate |
|
Moderate |
Airborne precautions for settings with uncertain or high residual risk
Where the verification of engineering responses are uncertain or the residual risk remains high, the following approach is proposed.
Modelling ventilation rates for limiting transmission risk in the context of the proposed quantum generation rate[46] for airborne COVID-19 [47] supports the conclusion that the COVID-19 ventilation rates should be not less that 59.7 L/s per person for an 8 hour exposure in a 100 seater waiting room, which (coincidentally) matches the WHO's previous recommendations for medium risk spaces and the CDC's adoption of that value for COVID-19 (see above).
Reducing the exposure time would reduce the required ventilation rate. 8h is assumed to keep staff and patients equally "safe". Obviously additional precautions are recommended for health care staff to control for repeated daily exposure.
A significant problem with the WHO and CDC recommendation of 60 L/s per patient (person) is that an individual's risk increases from 1% to nearly 40% as this criteria is applied to room populations decreasing from 100 persons.
To maintain a personal risk below 1% the following correction is advised:
<math>Q/n = 60 \times \bigl(A/n) </math> <math>\therefore Q = 60 \times \bigl(m^2 per person) </math>
Where Q is the ventilation rate <math>(L/s\cdot m^2)</math>, A is the floor area <math>(m^2)</math> and n is the maximum number of people in the space.
Air changes per hour (ACH) can be estimated with <math>ACH = Q \times 3.6 \div h </math>, where <math>h</math> is the ceiling height.
Assuming face masks reduce transmission by 40%, ventilation rates of 38 <math>L/s\cdot person</math> would be required to control airborne transmission. This sounds like a lot until the social distancing and decongestion measures required for droplet precautions are applied. under these conditions this equates to only 4.23 <math>L/s\cdot m^2</math> or between 4 and 5 ACH (assuming a 3.2m ceiling height). This should be easily achievable for naturally ventilated spaces and could be verified by measuring average indoor CO2 concentrations no greater than 170 PPM above outdoor.
Conclusion
Therefore, assuming ventilation systems in South Africa have been designed in accordance with the IUSS guidance, there should be little reason to change their configuration or pressurization unless general areas are repurposed as airborne precaution rooms. Risk assessments should be conducted for ICUs and COVID-19 wards immediately adjacent to public waiting areas or other high traffic areas, with corrective actions including but not limited to reducing occupancy times and rates for these areas and adjusting distancing rules.
Notes and References
- ↑ Liu, L., Li, Y., Nielsen, P. V., Wei, J. & Jensen, R. L. Short-range airborne transmission of expiratory droplets between two people. Indoor Air 1–11 (2016) doi:10.1111/ina.12314.
- ↑ https://www.who.int/news-room/commentaries/detail/modes-of-transmission-of-virus-causing-covid-19-implications-for-ipc-precaution-recommendations
- ↑ World Health Organization. Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19) 16-24 February 2020 [Internet]. Geneva: World Health Organization; 2020 Available from: source/coronaviruse/who-china-joint-mission-on-covid-19-final-report.pdf https://www.who.int/docs/default- source/coronaviruse/who-china-joint-mission-on-covid-19-final-report.pdf
- ↑ Wenzhao Chen, Nan Zhang, Jianjian Wei, Hui-LingYen, and Yuguo Li, “Short-range airborne route dominates exposure of respiratory infection during close contact,” medRxiv preprint, https://doi.org/10.1101/2020.03.16.20037291
- ↑ 5.0 5.1 5.2 Neeltje van Doremalen, Trenton Bushmaker, Dylan H. Morris, Myndi G. Holbrook, Amandine Gamble, Brandi N. Williamson, Azaibi Tamin, Jennifer L. Harcourt, Natalie J. Thornburg, Susan I. Gerber, James O. LloydSmith, Emmie de Wit, and Vincent J. Munster, “Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1,” The New England Journal of Medicine (2020), DOI: 10.1056/NEJMc2004973 [1]
- ↑ Faridi, S. et al. A field indoor air measurement of SARS-CoV-2 in the patient rooms of the largest hospital in Iran. Sci. Total Environ. 725, 1–5 (2020).
- ↑ Liu, Y. et al. Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitals. Nature (2020) doi:10.1038/s41586-020-2271-3.
- ↑ Fears SC, Klimstra WB, Duprex P, Hartman A, Weaver SC, Plante KS, et al. Persistence of severe acute respiratory syndrome coronavirus 2 in aerosol suspensions. Emerg Infect Dis. 2020 Sep [date cited]. https://doi.org/10.3201/eid2609.201806
- ↑ Robert Comparison of the Aerosol Stability of 2 Strains of Zaire ebolavirus From the 1976 and 2013 Outbreaks Robert J. Fischer, Trenton Bushmaker, Seth Judson, Vincent J. Munster J Infect Dis. 2016 Oct 15; 214(Suppl 3): S290–S293. Published online 2016 Oct 4. doi: 10.1093/infdis/jiw193 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5050463/
- ↑ Isao Arita, Kazunobu Kojima, and Miyuki Nakane, “Transmission of severe acute respiratory syndrome,” Emerging. Infectious Diseases 9 No. 9 (2003):1183-84, [2].
- ↑ 11.0 11.1 Lu, J., Gu, J., Li, K., Xu, C., Su, W., Lai, Z....Yang, Z. (2020). COVID-19 Outbreak Associated with Air Conditioning in Restaurant, Guangzhou, China, 2020. Emerging Infectious Diseases, 26(7), 1628-1631. https://dx.doi.org/10.3201/eid2607.200764.[3]
- ↑ How Coronavirus Infected Some, but Not All, in a Restaurant, Chang, K (2020) https://www.nytimes.com/2020/04/20/health/airflow-coronavirus-restaurants.html
- ↑ https://english.elpais.com/spanish_news/2020-06-17/an-analysis-of-three-covid-19-outbreaks-how-they-happened-and-how-they-can-be-avoided.html
- ↑ Park SY, Kim YM, Yi S, Lee S, Na BJ, Kim CB, et al. Coronavirus disease outbreak in call center, South Korea. Emerg Infect Dis. 2020 Aug [date cited]. https://doi.org/10.3201/eid2608.201274
- ↑ Mangili, A., & Gendreau, M. A. (2005). Transmission of infectious diseases during commercial air travel. Lancet (London, England), 365(9463), 989–996. https://doi.org/10.1016/S0140-6736(05)71089-8
- ↑ Olsen et al, N Engl J Med 2003; 349:2416-2422Transmission of the Severe Acute Respiratory Syndrome on Aircraft, DOI: 10.1056/NEJMoa031349 [4]
- ↑ CMAJ 2020 April 14;192:E410. doi: 10.1503/cmaj.75015 [5]
- ↑ 18.0 18.1 McKinney KR, Gong YY, Lewis TG. Environmental transmission of SARS at Amoy Gardens. J Environ Health. 2006;68(9):26-52.
- ↑ Bhowmick, G.D., Dhar, D., Nath, D. et al. Coronavirus disease 2019 (COVID-19) outbreak: some serious consequences with urban and rural water cycle. npj Clean Water 3, 32 (2020). https://doi.org/10.1038/s41545-020-0079-1
- ↑ Santarpia et al, “Transmission Potential of SARS-CoV-2 in Viral Shedding Observed at the University of Nebraska Medical Center,. medRxiv preprint (2020), [6]
- ↑ Po Ying Chia et al, 2020 (Preprint) “Detection of Air and Surface Contamination by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) in Hospital Rooms of Infective Patients,” medRxiv preprint (2020), https://doi.org/10.1101/2020.03.29.20046557 [7]
- ↑ Ong SWX, Tan YK, Chia PY, et al. Air, Surface Environmental, and Personal Protective Equipment Contamination by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) From a Symptomatic Patient. JAMA. 2020;323(16):1610–1612. doi:10.1001/jama.2020.3227
- ↑ Chin, A. W. H. et al. Stability of SARS-CoV-2 in different environmental conditions. The Lancet Microbe 0–4 (2020) doi:10.1016/s2666-5247(20)30003-3.
- ↑ Pyankov, O. V., Bodnev, S. A., Pyankova, O. G. & Agranovski, I. E. Survival of aerosolized coronavirus in the ambient air. J. Aerosol Sci. 115, (2018).
- ↑ 25.0 25.1 WHO 2020, Modes of transmission of virus causing COVID-19: implications for IPC precaution recommendations https://www.who.int/news-room/commentaries/detail/modes-of-transmission-of-virus-causing-covid-19-implications-for-ipc-precaution-recommendations
- ↑ Pan Y, Zhang D, Yang P, Poon LLM, Wang Q. Viral load of SARS-CoV-2 in clinical samples. Lancet Infect Dis. 2020;20(4):411-2.
- ↑ Kitajima et al,SARS-CoV-2 in wastewater: State of the knowledge and research needs,Science of The Total Environment,Volume 739,2020,139076,ISSN 0048-9697,https://doi.org/10.1016/j.scitotenv.2020.139076
- ↑ https://www.cdc.gov/sars/about/faq.html
- ↑ https://www.cdc.gov/sars/about/faq.html
- ↑ US-CDC,2005, https://www.cdc.gov/sars/guidance/i-infection/healthcare.html
- ↑ Interim Infection Prevention and Control Recommendations for Healthcare Personnel During the Coronavirus Disease 2019 (COVID-19) Pandemic (updated July 9, 2020)[8]
- ↑ CDC 2019, COVID-19 Overview and Infection Prevention and Control Priorities in Non-US Healthcare Settings https://www.cdc.gov/coronavirus/2019-ncov/hcp/non-us-settings/overview/index.html
- ↑ WHO 2009. WHO Policy on TB infection control in Health-care facilities, Congregate Settings and Households. https://apps.who.int/iris/bitstream/handle/10665/44148/9789241598323_eng.pdf?sequence=1
- ↑ CDC 2005, Guidelines for Environmental Infection Control in Health-Care Facilities (updated 2019)https://www.cdc.gov/mmwr/pdf/rr/rr5417.pdf
- ↑ WHO 2019, WHO Guidelines on tuberculosis infection prevention and control, 2019 update https://www.who.int/tb/publications/2019/guidelines-tuberculosis-infection-prevention-2019/en/
- ↑ Q: Does ASHRAE’s guidance agree with guidance from WHO and CDC? (ASHRAE 2020)[9]
- ↑ ASHRAE healthcare C19 guidance (ASHRAE 2020) [10]
- ↑ ASHRAE residential c19 guidance (ASHRAE 2020)[11]
- ↑ COVID 19 guidance for multifamily building owners-managers (ASHRAE 2020)[12]
- ↑ ASHRAE commercial C19 guidance (ASHRAE 2020)[13]
- ↑ ASHRAE Schools C19 guidance (ASHRAE 2020)[https://www.ashrae.org/file%20library/technical%20resources/covid-19/ashrae-schools-c19-guidance.pdf
- ↑ REHVA COVID-19 guidance document, April 3, 2020[14]
- ↑ Building Engineering Services (2014)[15]
- ↑ Government Notice R116 (17 Feb 2014)[16]
- ↑ SABS. SANS 10400-O : 2011 SOUTH AFRICAN NATIONAL STANDARD The application of the National Building Regulations Part O : Lighting and Ventilation. (2011).
- ↑ Noakes, C. J. & Sleigh, P. A. Mathematical models for assessing the role of airflow on the risk of airborne infection in hospital wards. J. R. Soc. Interface 6, S791–S800 (2009). https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2843948/
- ↑ Zhao, Dai, preprint 2020, Association of infected probability of COVID-19 with ventilation rates in confined spaces: a Wells-Riley equation based investigation https://doi.org/10.1101/2020.04.21.20072397