Healthcare Technology: Difference between revisions
No edit summary |
No edit summary |
||
| (31 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
=Background= | |||
[[Category: | |||
==Health technology context== | |||
Health Technology covers a wide range of apparatus, consumables, devices, equipment and instruments that would require many volumes of documents to cover effectively. This document, as part of the broader IUSS Norms and Standards project, aims to look at the key elements of Health Technology and its management as it manifests in healthcare facilities. The document specifically aims at framing the subject of Health Technology within the infrastructure development and operations domain. | |||
The various definitions of Health Technology are listed in the Glossary but it is important to note that this document focuses on durable medical equipment and related consumables used in healthcare facilities. Hospital plant and machinery, normally associated with the building and most often installed as part of the building, are dealt with in various other documents in the IUSS document bouquet, either under the respective hospital engineering disciplines or under the respective clinical service area. | |||
==Application of the Guidelines== | |||
This document, published under the Health Facility guides, would be useful for practitioners of Health Technology Management both at the level of an individual facility and for groups of facilities (for example, in a district) and at any level of care. It would also be of great value for HTM practitioners and facility managers in general who might lack the first-hand experience of this relatively specialised field. In many cases the guidelines show the way towards developing specific and applicable solutions rather than being prescriptive. This approach is necessary to ensure that they remain sufficiently generic to be applied across a broad spectrum of scenarios. | |||
The document would also be of interest to health infrastructure professionals who might need specific insight into the domain of Health Technology and its management, recognising that technology and infrastructure are closely inter-related and will become ever more so in future healthcare systems. | |||
==Associated process and related documents== | |||
This document should be considered in the context of the solid platform provided by the '''Health Technology Policy''' and the '''Health Facilities Planning''' Directorates of the National Department of Health. This includes the '''Framework for Health Technology Policies''' which outlines the vision of a National Health Technology System; the draft Health Technology Management Policy document which inter alia outlines the organisational and structural requirements at all levels of governance pertaining to such a system; the '''Health Technology Strategy''' which outlines specific objectives and activities, with associated timeframes, for establishing a National Health Technology System; the '''Report of the Interim Steering Committee on Health Technology Assessment''', which underlines the importance of having formal processes in place to assess issues of health technology cost-effectiveness, access, service-fit and social impact, amongst other “evaluative dimensions”; and lastly the draft '''Medical Device Regulations''' and associated background documents which – in their final form – will be promulgated under the South African Health Products Regulatory Agency (SAHPRA) Act . | |||
=Life cycle of Health Technology= | |||
==Planning== | |||
===Introduction=== | |||
Planning and budgeting are often considered jointly since planning – for it to be effective – needs to take place within the context of policy, financial, and other constraints. '''Box 1''' shows the overall process. | |||
BOX 1: '''The planning and budgeting process''' ('''start-up''') | |||
{| class="wikitable" | |||
|+ | |||
!Steps | |||
!'''People responsible''' | |||
!'''Actions''' | |||
|- | |||
|Plan and budget within the framework of guidance and direction from the national level. | |||
|Health service managers at national level in consultation with managers at other levels. | |||
|'''Framework requirements''' | |||
*follow regulations and standards set by government; | |||
*develop a Healthcare Technology Policy including decisions on standardisation, maintenance provision, finances for HTM activities, and the organisational structure for an HTM service; | |||
*define the overall “Vision” for healthcare delivery at each level of the health service; | |||
*develop “Standard Equipment Lists” which define the essential equipment stock for the healthcare to be delivered at each level; | |||
*use “Generic Equipment cifications” for acquisition ofequipment; | |||
*develop good policies for purchasing, donations, replacement, and disposal of equipment; and participate in the planning, equipping and operation of health facilities. | |||
|- | |||
| rowspan="2" |Increase the availability of planning skills for equipment at all service levels, by developing planning “tools” through one-off exercises. | |||
|HTM working groups and sub-groups. | |||
Finance officers. | |||
|'''Knowing where one is starting from''' | |||
*establish an Equipment Inventory to keep up-to-date records of the current equipment stock; | |||
*estimate the equipment stock values; | |||
*define the usage rates of equipment-related consumable items so that realistic estimates can be made of the finances required for equipment accessories, consumables, and spare parts; and | |||
*set up budget lines to record and monitor expenditure on all the different equipment activities. | |||
|- | |||
|Health management teams. | |||
<br />HTM working groups and sub-groups. | |||
|'''Knowing where one is headed''' | |||
*develop a library of literature and resources which will help with equipment planning and budgeting; | |||
*adapt the vision for healthcare delivery at their service level; | |||
*adopt good policies for purchasing, donations, replacement, and disposal of equipment; | |||
*adapt the Standard Equipment List for their service level; | |||
*develop generic equipment specifications and technical and environmental data; and estimate human resource requirements and associated “acquisition” and capacity building. | |||
|- | |||
|Ensure realistic estimates are made for all equipment-related allocations at all service.levels, by using budgeting “tools” to calculate expenditures required. | |||
|HTM working groups and sub-groups. | |||
<br />HTM managers and their teams. | |||
|'''Capital budget calculations''' | |||
*calculate expenditure requirements for replacement items; | |||
*calculate expenditure requirements for new purchases; | |||
*calculate expenditure requirements for support activities linked to purchases and donations; | |||
*calculate expenditure requirements for pre-installation work; and | |||
*calculate expenditure requirements for major rehabilitation work. | |||
|- | |||
|Ensure realistic estimates are made for all equipment-related allocations at all service levels, by using budgeting “tools” to calculate expenditures required. | |||
| | |||
HTM managers and their teams. | |||
Heads of section. | |||
HTM working groups and sub-groups. | |||
|'''Recurrent budget calculations''' | |||
*calculate recurrent expenditure requirements for maintenance; | |||
*calculate recurrent expenditure requirements for consumable operating costs; | |||
*calculate recurrent expenditure requirements for administrative expenses; and | |||
*calculate recurrent expenditure requirements for ongoing training. | |||
|- | |||
|Use the tools to make long-term plans and budgets | |||
| | |||
HTM working groups and sub-groups. | |||
|'''Long-term planning''' | |||
*establish an Equipment Development Plan covering the priorities for equipment needs across their service level over time; | |||
*establish an Equipment Training Plan to cover the ongoing rolling programme of training required in relation to equipment activities; | |||
*establish a Core Equipment Expenditure Plan which prioritises equipment spending across the facility over the long term; and | |||
*establish a Core Equipment Financing Plan indicating possible sources of funds and related options. | |||
|- | |||
|Review the plans and budgets annually, and monitor progress in order to improve planning and budgeting. | |||
| | |||
HTM teams. | |||
HTM working groups | |||
and sub-groups. | |||
|'''Annual planning''' | |||
*update the Equipment Inventory; | |||
*update the Equipment Development Plan; | |||
*update the Equipment Training Plan; | |||
*cost the capital and recurrent requirements for the current year, and update the Core Equipment Expenditure Plan and Core Equipment Financing Plan; and | |||
*prioritise across their service level to obtain the annual purchase activities, annual rehabilitation activities, annual corrective activities, annual training activities, and thus the annual equipment budget. | |||
|- | |||
|Review the plans and budgets annually, and monitor progress in order to improve planning and budgeting. | |||
| | |||
HTM working groups. | |||
Heads of department and HTM managers. | |||
Health management | |||
teams. | |||
|'''Monitoring progress''' | |||
*ensure annual plans are implemented; | |||
*study the implications arising from planning and budgeting; | |||
*request help for any deviations from plans such as emergency purchases, maintenance and consumable contingencies; | |||
*monitor actual expenditure against allocations; | |||
*seek the funding identified; | |||
*consider linking allocation of budgets to whether departments achieve their performance targets; | |||
*monitor progress through establishing planning and budgeting tools and associated indicators; and | |||
*ensure that the information generated is used to improve stock control, training, procurement, utilisation, etc. | |||
|} | |||
===Service and related technology planning=== | |||
For healthcare technology to be managed effectively, a clear idea of healthcare delivery goals and targets is needed, as well as the context in which the technology is operating. Equipment should not be viewed in isolation – it is there for a purpose, and must be managed according to set objectives. For effective planning, access to a wide range of information and reference materials is needed, as well as a clear vision of the direction in which the health service is headed, plus identification of what equipment is required to help achieve the health service goals. | |||
To inform the technology part of the debate, the HTM working group (at each level) should consider the equipment implications of the healthcare interventions suggested, and then offer technical advice to their health management team. '''Box 2''' and '''Checklist 1''' show some of the issues that the national, province/district- and facility level HTM working groups, respectively, should consider. | |||
BOX 2: '''Baseline information on medical devices''' | |||
{| class="wikitable" | |||
|+ | |||
|'''Medical device situation''' | |||
|'''Considerations''' | |||
|'''Result''' | |||
|- | |||
| | |||
*Availability and condition of medical devices (including type, number, location and physical condition). | |||
*Status of electrical, water, and waste disposal systems related to medical device use. | |||
| | |||
*Medical equipment inventory including status and condition. | |||
*Current health technology management infrastructure (or lack thereof). | |||
| | |||
*Facility map. | |||
*Medical equipment inventory (quantitative and qualitative). | |||
*Outline of health technology management infrastructure. | |||
|} | |||
CHECKLIST 1: '''Equipment considerations for vision at national level''' | |||
{| class="wikitable" | |||
|+ | |||
!Issues | |||
!Example | |||
|- | |||
|What expansion of services is necessary or feasible? | |||
| | |||
*What should be the role of a hospital (central, referral, district) in terms of the interventions and procedures to be carried out? What does this mean in terms of equipment availability? | |||
*What type of care can be offered by rural, district or town health centres? Can any types of care be transferred over to them? What does this mean in terms of equipment availability? | |||
*It may be best to locate certain specialised services (such as intensive-care units) only at certain hospitals. Some specialised services, such as radiotherapy, may only ever be offered at national level. With pressures to reduce costs, improve efficiencies, and possibly to reduce staff numbers, can service provision be rationalised? Is expansion based only on needs that can be realistically met? | |||
|- | |||
|What are the implications in terms of staff, skills, resources, patient referral networks? | |||
| | |||
*Introducing a new service has knock-on implications for human, material and financial resources. For example, should eye instruments be bought for a facility if there is no eye surgeon, or prospects of one becoming available? Or if the referral system is such that dialysis is only undertaken and supported at a central facility, one should think carefully before placing dialysis machines at other further locations. However, if such expanded “satellite” services are required, then all the resources required to ensure effective service provision should be identified, provided and maintained. | |||
|- | |||
|Are desired expansions financially affordable? | |||
| | |||
*Although many hospitals may ideally wish to have fluoroscopy facilities (for example), at a cost of approximately $500 000 per suite, is this a feature each hospital can necessarily invest in? | |||
|- | |||
|Do the services suggested fit into the overall health service in the country? | |||
| | |||
*Is it possible to develop a vision which fits in with the other health service provider organisations? | |||
|} | |||
===Planning and budgeting when starting out=== | |||
'''''Box 3''''' shows the minimum requirements for a scenario for an HTM system in its infancy, where the initial focus is not on long-term forward planning, but concentration on planning and budgeting on a yearly basis. As the system matures, other elements for forward planning can be added. | |||
Box 3: '''Minimum planning and budgeting requirements''' | |||
{| class="wikitable" | |||
|+ | |||
|'''Planning and budgeting element''' | |||
|'''If just starting out''' | |||
|- | |||
|1. Equipment inventory | |||
2. Stock value estimates | |||
3. Budget lines for equipment expenditures. | |||
4. Usage rates for equipment-related consumable items. | |||
5. Reference materials. | |||
6. Developing the vision of service delivery for each facility type. | |||
7. Standard Equipment Lists. | |||
8. Purchasing, donations, replacement, and disposal policies. | |||
9. Generic equipment specifications and technical data. | |||
10. Capital budget calculations. | |||
11. Recurrent budget calculations. | |||
12. Equipment development plan. | |||
13. Equipment training plan. | |||
14. Core equipment expenditure plan. | |||
15. Core equipment financing plan. | |||
16. Annual equipment planning and budgeting. | |||
17. Monitoring progress. | |||
|1. Essential to have. | |||
2. Useful to carry out this exercise later on when rough estimates needed for long-term forward planning. | |||
3. This alteration to budget layout can be done later, but it will help with analysis. | |||
4. Useful to do this exercise as it helps with calculation of specific (annual) estimates. | |||
5. These can be developed over time. | |||
6. Should have an understanding of this, even if full exercise not undertaken. | |||
7. Initially, list of urgent equipment needs drawn up by departments can be used. Later on, Standard Equipment Lists obtained elsewhere can be used. | |||
8. Essential to have. | |||
9. Initially learn from others. Later, develop own. | |||
10. Initially learn how to make specific (annual) estimates; only learn the rough estimation methods when undertaking long-term planning. | |||
11. Initially learn how to make specific (annual) estimates. Only learn the rough estimation methods when undertaking long-term planning. | |||
12. Use the basic equipment development planning process only, and only apply it to the short term. | |||
13. Develop a straightforward one for the short term | |||
14. Initially only plan annually (see below). | |||
15. Initially only plan annually (see below). | |||
16. Create annual actions plans and an equipment budget showing income and expenditure. | |||
17. Undertake the basic elements only – progress with annual plans and tools, coping with emergencies, providing feedback. | |||
|} | |||
==Needs assessment== | |||
===Standard lists of equipment=== | |||
Once the vision for the direction of health service delivery has been developed, the healthcare interventions and procedures to be offered will be known. Based on this information, the Essential Service Packages can be developed; these will translate the vision into: | |||
*human resource requirements, and training needs; | |||
*space requirements, and facility and service installation needs; and | |||
*equipment requirements. | |||
The tool used in the process of defining what equipment is needed for each healthcare intervention is the '''Standard Equipment List'''. This is: | |||
*a list of equipment typically required for each healthcare intervention (such as a healthcare function, activity, or procedure). For example, health service providers might list all equipment required for eye-testing, delivering twins, undertaking fluoroscopic examinations, or for testing blood for malaria; | |||
*organised by activity space or room (such as reception area or treatment room), and by department; | |||
*developed for every different level of healthcare delivery (such as district, province) since the equipment needs will differ depending on the vision for each level; | |||
*usually made up of '''everything''' including furniture, fittings and fixtures, in order to be useful for planners, architects, engineers and purchasers, and | |||
*a tool which allows healthcare managers to establish if the Vision is economically viable. | |||
The Standard Equipment List must reflect the level of technology of the equipment. It should describe only technology that the facility can sustain (in other words, equipment which can be operated and maintained by existing staff, and for which there are adequate resources for its use). For example a department could have: | |||
*an electric suction pump or a foot-operated one; | |||
*a hydraulic operating table or an electrically controlled one; | |||
*a computerised laundry system or electro-mechanical machines; and | |||
*disposable syringes or re-usable/sterilisable ones. | |||
It is important that any equipment suggested: | |||
*can fit into the rooms and space available. Reference should therefore be made to any building norms defining room sizes, flow patterns, and requirements for water, electricity, light levels and so on; | |||
*has the necessary utilities and associated plant (such as the power, water, waste management systems) available for it on each site - if such utilities are not available, it is pointless planning to invest in equipment which requires these utilities in order to work; and | |||
*can be operated and maintained by existing staff and skill levels, or for which the necessary training is available and affordable. | |||
====Usefulness of the standard equipment lists==== | |||
A Standard Equipment List is an aid to the planning process. In order to plan what equipment to purchase, awareness of any shortfalls in equipment is needed. To determine such shortfalls, the Equipment Inventory needs to be compared with the Standard Equipment List. This will indicate whether any equipment is currently missing or needs to be purchased. Thus, the Standard Equipment List will assist in determining what equipment is: | |||
*necessary; | |||
*surplus; | |||
*extravagant; and | |||
*missing | |||
in relation to the Vision for the service. | |||
Responsibility for developing the Standard Equipment Lists varies from country to country. It is most important that this task is undertaken by a multidisciplinary team, so that decisions benefit from the skills and views of many disciplines, not just one or two. The health service planners at central/national level should consider developing Standard Equipment Lists in collaboration with staff from each level of the service, as indicated in '''''Box 4''''' below. | |||
{| class="wikitable" | |||
|'''Who/which level?''' | |||
|'''Takes what action?''' | |||
|- | |||
|HTM working group at each level | |||
|Organises special meetings of different types of staff to work on the Standard Equipment List. Then reports back to the Health Management Teams. | |||
|- | |||
|National level | |||
|Takes the '''first step''' and runs specific exercises to establish the Standard Lists of Equipment for each clinical and support area, at each operational level. | |||
|- | |||
|Province/district level | |||
|Takes the '''second step''' and adjusts the list on a regional/district basis to cover local variations. | |||
|- | |||
|Facility level | |||
|Takes the '''third step''' and assesses: | |||
- how they can provide the healthcare interventions; and | |||
- what numbers of equipment they require depending on how they organise their work. | |||
Organisational decisions influence the quantity of equipment. For example, the timing of clinics can reduce or increase the workload in the laboratory. Before ordering new equipment, its level of use will need to be assessed. | |||
|} | |||
===Equipment development plan=== | |||
Especially for an HTM system that is not mature, it is essential to have important to have [BvR1] an equipment development plan; one suggestion for the development planning process is presented in '''''Flowchart 1''''' below. | |||
Flowchart 1: '''The basic equipment development planning process''' | |||
[[File:Flowchart 1 - The basic equipment development planning process.PNG|thumb|none|720x720px]] | |||
====Prioritising and appraisal of options==== | |||
It is equally important, in the context of needs assessment, to critically examine the process and its outputs and outcomes. '''''Checklist 2''''' below poses some key questions for consideration by the appropriate stakeholder/s. | |||
Checklist 2: '''Key questions for prioritising and appraisal of options''' | |||
{| class="wikitable" | |||
|'''Impact''' | |||
*Which changes would have the greatest positive impact in meeting needs? | |||
*Do the identified needs relate to a local or a national priority (e.g., maternal and child health, HIV/AIDS, etc.)? | |||
*What would be the implications of not addressing the needs? | |||
|- | |||
|'''Changeability''' | |||
*Which things can be changed and effectively improved? | |||
*What evidence is there of effective interventions? | |||
*Can negative impacts be stopped or reduced? | |||
*Are there national or local, professional or organisational policies that set out guidelines on what should be done (e.g. national frameworks, national guidance, etc.)? | |||
|- | |||
|'''Acceptability''' | |||
*Which of the options for change are likely to be most acceptable to the health service providers, to the target population, and to the managers? | |||
*What might be the “knock-on” effects, or unintended consequences, of making a change? | |||
|- | |||
|'''Resource feasibility''' | |||
*Which resources are required to implement the proposed changes? | |||
*Can existing resources be used differently? | |||
*Which resources will be released if ineffective actions are stopped/changed (e.g., proper management of health technology, etc.)? | |||
*Are there other resources available which have not been given prior consideration (e.g., income generation of laboratory services, consideration of public-private partnerships, assistance from NGOs, etc.)? | |||
*Which of the actions will achieve the greatest impact for the resources used? | |||
|} | |||
===Consumables=== | |||
Consumables are a major contributor to equipment-related cost of ownership, and form a significant portion of recurrent and operational expenditure. It is therefore important to establish the consumables needs so that adequate provision is made and service delivery is not interrupted. '''''Flowchart 2''''' below suggests a process to facilitate the needs assessment related to equipment-related consumables. | |||
FLOWCHART 2: Establishing usage rates and requirements for equipment-related consumable items | |||
[[File:Flowchart 2 -Establishing usage rates and requirements for equipment-related consumable items.PNG|thumb|none|720x720px]] | |||
==Asset management== | |||
===Inventory=== | |||
It is essential that an inventory (asset register) of medical equipment is maintained. However, it is both costly and time-consuming to include each and every medical device in an asset register. For those items in an asset register, the following information should be captured and verified: | |||
*Equipment type (an international nomenclature system such as the Universal Medical Device Nomenclature System (UMDNS) should be used so that all institutions use a common name for the same type of device). | |||
*Make/manufacturer. | |||
*Model. | |||
*Serial number. | |||
*Date of acquisition. | |||
*Price paid (include any costly accessories). | |||
*Supplier details (name, address, contact person, contact details). | |||
*Location – department or ward where the unit is used. | |||
The responsibilities, activities and role-players relating to equipment inventory are shown in '''''Box 5.''''' | |||
Box 5: '''Inventory-related responsibilities, activities and actors''' | |||
{| class="wikitable" | |||
|'''Body''' | |||
|'''Responsibility''' | |||
|'''Activity''' | |||
|'''People involved''' | |||
|- | |||
|HTM Service | |||
|Creates and updates the Equipment Inventory. | |||
|Organises the gathering of inventory data. | |||
|Either by: | |||
*facility staff for their own facility; | |||
*district staff for the facilities in their district; | |||
*central staff for the health service as a whole; and | |||
*using specialist help. | |||
|- | |||
|Inventory team | |||
| | |||
|Visits each department in the health facility, and: | |||
*looks in all rooms, cupboards, etc.; | |||
*physically checks all equipment for the details required; and | |||
*fills in the Equipment Inventory Record Sheets. | |||
If existing records are available: | |||
*modifies or expands the information as necessary to cover new items; | |||
*fills in any gaps; | |||
*corrects entries; andupdates data in order to make the Equipment Inventory as accurate as possible. | |||
|Due to the workload and knowledge required, it is useful for the team to be made up of: | |||
*two maintenance staff (from the relevant HTM team); | |||
*a senior equipment user from the facility; and | |||
*a member of staff from the department being assessed (who changes as team moves from department to department). <br /> | |||
|- | |||
|HTM teams | |||
|Compile the Equipment Inventory. | |||
Make hard copies. | |||
| | |||
*Enter the data gathered, either onto an inventory card or a computer screen, for each individual device. | |||
*Create summaries, prepare and print out hard copies. | |||
*Provide a copy of the Equipment Inventory.to the Stores Controller for inclusion in the General Inventory held by stores. | |||
|Make use of trained technical staff and secretarial/computing support to assist with data entry. | |||
|- | |||
|Central-level HTM team | |||
|Develops the Equipment Inventory as an active (regularly updated) resource. Analyses the Equipment Inventory for planning purposes. | |||
| | |||
*Uses computer-based tools required for this purpose (for example, word-processing spreadsheets or specific commercial inventory products which staff have been trained on. | |||
|Makes use of support from staff trained in keeping computerised records. | |||
|} | |||
===Asset management system=== | |||
Proper asset management requires an appropriate information system which can be used to maintain data on those items that have been included in the asset register. | |||
Equipment audits provide a snapshot of equipment status in a facility or groups of facilities, and can be aggregated to reveal the situation at national level. Audit data also serves to inform decision making related to needs assessment, asset management, maintenance strategies, replacement planning, and so on. | |||
Also, once an inventory system is up and running, it will be necessary to periodically obtain estimates of total equipment stock values – this is used, for example, in benchmarking the total expenditure on maintenance. '''''Flowchart 3''''' below shows the process for obtain such estimates. | |||
====Asset management software==== | |||
It is essential to adopt and standardise on an appropriate asset management information system. There are a number of such systems available; serious consideration should be given to open-source solutions and/or or solutions which are cost-effective and sustainable, requiring minimal support. | |||
Flowchart 3: '''How to estimate total equipment stock values''' | |||
[[File:Flowchart 3 - How to estimate total equipment stock values.PNG|thumb|none|720x720px]] | |||
==Budgeting & financing== | |||
===Estimates of budget lines for equipment expenditure=== | |||
It is essential to have budget lines for health technologies/medical equipment in national, province, district and facility budgets. A process for developing budget lines is shown in '''''Box 6'''''. | |||
Box 6: '''Process for developing budget lines for equipment expenditure''' | |||
{| class="wikitable" | |||
|'''People responsible''' | |||
|'''Action''' | |||
|- | |||
|Finance officers, at all levels of the health service (central, province, district, facility) | |||
|Establish different budget lines (sub-divisions) as itemised below: | |||
a. capital funds to cover equipment replacement (depreciation); | |||
b. capital funds to cover additional new equipment requirements; | |||
c. capital funds to cover support activities which ensure equipment purchases can be used (installation, commissioning, and initial training); | |||
d. capital funds to cover pre-installation work for equipment purchases; | |||
e. capital funds to cover major rehabilitation projects; | |||
f. recurrent funds to cover equipment maintenance costs, including spare parts, service contracts, and minor works; | |||
g. recurrent funds to cover equipment operational costs, including consumable items and worn out accessories; | |||
h. recurrent funds to cover equipment-related administration, including energy requirements; and | |||
i. recurrent funds to cover ongoing training requirements. | |||
|- | |||
|HTM working groups | |||
|Start using these budget lines to analyse how money is allocated and spent for equipment purposes. | |||
|- | |||
|Health service providers | |||
|Ensure that budgets are presented by cost centre so that it is clear what allocations are made between national, provincial, district and facility levels. In this way, it can be seen what money is spent on equipment activities at each level of the health service. Lobby other bodies involved (such as Ministries of Finance, Public Works) to also show equipment expenditures to establish what is allocated by other agencies for equipment activities in the health service. | |||
|} | |||
The high-level budgets thus obtained can then be “unpacked” into more detailed line expenditures, such as shown in '''''Table 1''''' below, with projections covering 3-5-year budget cycles. | |||
===Estimates of maintenance costs for forward planning=== | |||
An important – and often neglected – expenditure category is that of maintenance, be it for medical equipment or healthcare technologies and infrastructure in general. '''''Box 7''''' unpacks the maintenance line item to indicate the various categories of maintenance-related expenditure for medical equipment, while '''''Flowchart 4''''' suggests a process for estimating maintenance costs as part of planning. | |||
Table 1: '''Example of a core equipment expenditure plan''' | |||
{| class="wikitable" | |||
| rowspan="2" |'''Capital expenditure''' | |||
| colspan="2" |'''Short term''' | |||
| colspan="2" |'''Medium term''' | |||
|- | |||
|2012 | |||
|2013 | |||
|2014 | |||
|2015 | |||
|- | |||
|Replacement | |||
| rowspan="5" |''Use calculations for rough estimations (see Note below)'' | |||
| | |||
| | |||
| | |||
|- | |||
|New equipment | |||
| | |||
| | |||
| | |||
|- | |||
|Support activities linked to purchases | |||
| | |||
| | |||
| | |||
|- | |||
|Pre-installation | |||
| | |||
| | |||
| | |||
|- | |||
|Rehabilitation | |||
| | |||
| | |||
| | |||
|- | |||
|Sub-total | |||
| | |||
| | |||
| | |||
| | |||
|- | |||
|'''Recurrent expenditure''' | |||
| | |||
| | |||
| | |||
| | |||
|- | |||
|Equipment maintenance | |||
| rowspan="4" |''Use calculations for rough estimations (see Note below)'' | |||
| | |||
| | |||
| | |||
|- | |||
|Consumables | |||
| | |||
| | |||
| | |||
|- | |||
|Administration | |||
| | |||
| | |||
| | |||
|- | |||
|On-going training | |||
| | |||
| | |||
| | |||
|- | |||
|Sub-total | |||
| | |||
| | |||
| | |||
| | |||
|- | |||
|'''Total expenditure''' | |||
| | |||
| | |||
| | |||
| | |||
|} | |||
Note: Initially, rough estimates are used for the short- and long-term overview when preparing this Core Equipment Expenditure Plan. During annual planning the estimates are revised, using calculations for specific requirements, to obtain the Annual Equipment Budget. The experience gained from that annual revision process may mean that the long-term estimates in this Core Equipment Expenditure Plan may have to be altered, so that they are more realistic. | |||
Box 7: '''Elements of annual maintenance budgets''' | |||
{| class="wikitable" | |||
| | |||
'''I. Planned budgets:''' | |||
These allocate funds for anticipated maintenance costs, which can be derived from the following main areas of expenditure: | |||
a) spare parts – which are required regularly, determined from previous experience and any planned remedial work; | |||
b) spare parts – which are required according to planned preventive maintenance (PPM) schedules and timetables; | |||
c) maintenance materials – which are required regularly, determined by previous experience and any planned remedial work; | |||
d) maintenance materials – which are required according to PPM schedules and timetables; | |||
e) service contracts – required for any planned remedial work; | |||
f) service contracts – for breakdowns which are likely to be required, determined from previous experience; | |||
g) service contracts – required for PPM of complex equipment; | |||
h) calibration of workshop test equipment; | |||
i) replacement of tools at the end of their life; | |||
j) office material; and | |||
k) any increased maintenance requirements brought about by planned new equipment purchases under the capital expenditure budget. | |||
Note: there will be other elements which may fall under other budgets. These could include: | |||
*other administrative costs which are included in budgets held by other departments; | |||
*major repair works – in some cases the planned rehabilitation of equipment which rquires major work with the purchase of substantial amounts of materials or contracts (the large sums of money required for such projects may have to fall under the capital budget); and | |||
*pre-installation work (such as site-preparation); this often falls under capital funds as it is linked to specific purchases. | |||
|- | |||
| | |||
'''II. Contingency budgets:''' | |||
In addition to planned budgets, contingency budgets also exist. These allocate funds for unplanned maintenance work, such as emergencies, or sudden breakdowns which could not be predicted. | |||
|} | |||
Flowchart 4: '''How to make rough estimates of maintenance costs for forward planning''' | |||
[[File:Flowchart 4 - How to make rough estimates of maintenance costs for forward planning.PNG|thumb|none|720x720px]] | |||
===Estimates of consumable operating costs for forward planning=== | |||
In Section A2 (Needs assessment) the importance of consumables for proper functioning and utilisation was highlighted. Since medical devices cover such a wide spectrum, the related consumables require insight and good management. A number of alternative approaches are suggested: | |||
i. Consumption depends on the type of equipment used, the service provided, and how many patients are seen. Therefore, a rough estimation of consumable operating costs can be obtained by evaluating past usage rates/expenditures, and comparing these with expected patient loads and specific equipment usage rates per intervention. | |||
ii. If the equipment is part of a “closed” purchasing system, the consumables are only made by one manufacturer and one is limited to a single supplier; this monopoly often increases the consumable costs. If the equipment is part of an “open” purchasing system, anyone can supply the consumables and different manufacturers’ consumables could be used; this competition brings down costs of consumable. Costs can also be kept down by using items which can be sterilised/re-used. | |||
iii. Consumable operating costs vary according to equipment type, and can be expressed as a percentage of purchase cost or stock value, as shown by the examples below. But as the majority of equipment is likely to be technology that has low to medium consumable costs, one could use averages of 3% of the stock value for equipment with low consumable usage rates, and for others as shown in '''''Table 2'''.'' | |||
Table 2: '''Rough estimates of consumables’ operating costs for forward planning''' | |||
{| class="wikitable" | |||
|'''Description''' | |||
|'''Consumable cost per year (relative to original purchase cost)''' | |||
|- | |||
|Equipment with high consumable operating costs, such as: | |||
*Haemodialysis machine | |||
*Automatic biochemical analyser | |||
*Automatic haematology analyser | |||
*Electrolyte analyser | |||
*Blood gas analyser | |||
|70-120% | |||
|- | |||
|Equipment with medium consumable operating costs, such as: | |||
*Conventional x-ray machine | |||
*Anaesthesia machine | |||
| | |||
30% | |||
20% | |||
|- | |||
| | |||
*ECG recorder, three-channel | |||
*Ultrasound, medical/obstetric | |||
*Ventilator, ICU | |||
*Physiological monitor | |||
*EEG machine | |||
|15-25% | |||
|- | |||
| | |||
*Autoclave, steam | |||
*Incubator, baby, ICU | |||
|10-15% | |||
5-15% | |||
|- | |||
|Equipment with low consumable operating costs, such as: | |||
*Centrifuge, electrical | |||
*Suction pump | |||
| | |||
5% | |||
2-5% | |||
|- | |||
| | |||
*Delivery bed | |||
*Operating theatre lamp | |||
*Slit lamp | |||
*Operating microscope | |||
*Water bath | |||
|1-2% | |||
|} | |||
A different calculation is required when making specific or annual estimates. Annual operating budgets should be based on more exact estimates. These are not always easy to predict since epidemics, outbreaks, or surges in workload cannot, in most cases, be anticipated. | |||
Generally with experience, and where standardisation of equipment is in place, the projection for equipment consumables and spare accessories becomes more predictable. | |||
====Specific or annual estimates of consumable operating costs==== | |||
It is equally important to make provision for consumables on an annual basis. '''''Flowchart 5''''' suggests how this could be done. | |||
FLOWCHART 5: How to make specific or annual estimates of consumable operating costs | |||
[[File:Flowchart 5 - How to make specific or annual estimates of consumable operating costs.PNG|thumb|none|720x720px]] | |||
===Estimates of equipment replacement costs=== | |||
Equipment replacement needs to be provided for, especially in the case of complex and expensive equipment, to ensure that adequate resourcing is available at the appropriate time to ensure continuity – or at least minimal disruption – of service delivery. '''''Flowchart 6''''' below suggests a process to establish estimates for replacement costs. | |||
===Rough estimates of equipment-related administrative costs for forward planning=== | |||
The administrative costs associated with medical equipment are also seldom considered separately, since they are hidden within general overheads. Different countries suggest alternative approaches: | |||
i. Administrative costs are a small percentage of any operating budget; for example: | |||
*the biggest percentage expense is for staff, taking 50-55%; | |||
*supplies/spares take 35-45%; and | |||
*administration takes only 10-20%. | |||
Thus an equipment-user department could use an average of 15% of <s>their</s> <ins>its </ins>total operating budget for administrative costs. | |||
ii. For HTM teams and clinical engineering service maintenance workshops, their administrative needs are not much higher than other administrative units in health facilities. Therefore, a reasonable estimate for the administrative costs[1] for HTM teams could be calculated by taking 10-20% of their total operating budget. | |||
iii. A starting point is to use 5% of the equipment stock value to cover equipment-related administrative costs. | |||
FLOWCHART 6: How to make rough estimates of replacement costs for forward planning | |||
[[File:Flowchart 6 - How to make rough estimates of replacement costs for forward planning.PNG|thumb|none|720x720px]] | |||
As in the case of consumable-related costs, it is important to determine the annual equipment-related administrative costs ('''Flowchart 7'''). | |||
FLOWCHART 7: '''How to make specific estimates of assorted equipment-related administrative costs''' | |||
[[File:Flowchart 7 - How to make specific estimates of assorted equipment-related administrative costs.PNG|thumb|none|720x720px]] | |||
==Requisitioning== | |||
The following checklists cover three commonly encountered scenarios: | |||
Checklist 3: '''Request to purchase new equipment that is not yet in use at the institution''' | |||
a. Why is it essential to have this equipment and how will it enhance the present patient care. How was this function performed before and up until this request? | |||
b. Statistics of the patients who will be treated with this equipment. | |||
c. Is this equipment in line with the norms of the service delivery – level of care –of the institution? | |||
d. Accessibility to similar equipment or services in a close proximity to the Institution. | |||
e. Is suitable accommodation available to install the equipment (building and facilities). Is the structure suitable to carry the additional mass? This to be confirmed by the Engineering Services Manager, in writing. | |||
f. Are there engineering services available to operate the equipment and has the availability of the service been confirmed in writing by the Engineering Services Manager, for instance: | |||
*sufficient water pressure and flow (hot and cold); | |||
*sufficient water pressure and flow (hot and cold); | |||
*medical gas and compressed air (at the correct pressures and flow); | |||
*sufficient power at the correct voltage and current levels; | |||
*if required, a UPS system with a sufficient capacity; | |||
*a power line-conditioning unit for sensitive electronic equipment; | |||
*if required (for example autoclaves), is steam, condensate return and drainage available; and | |||
*if required, is the ventilation and air-conditioning sufficient. | |||
g. Are proper specifications available from the HTM unit or is suitable equipment available from an approved period tender? State the tender and the item numbers. | |||
h. Confirmation must be obtained from the Institution that they have a budget (sufficient funds) to pay for a service contract (where called for), as well as consumables and preventive and corrective maintenance of the new equipment. | |||
i. Will not having the equipment in any way compromise patient care or safety? | |||
Checklist 4: '''Request to replace existing equipment''' | |||
a. Inventory of similar equipment available in the Institution. | |||
b. Inventory of how many pieces of the type of equipment are functional. | |||
c. Inventory of how many pieces of equipment are not functional and the reason thereof. | |||
d. Is this equipment in line with the norms of service delivery – level of care – of the institution? | |||
e. Reason for the condemning of the equipment, supported by a Condemning Certificate. | |||
f. Where the replacement is a fixture for instance an x-ray machine or processor, what engineering or structural changes will be required? | |||
g. Does the Institution have sufficient funds to purchase the equipment requested? | |||
h. Does the Institution have a budget, sufficient funds to pay for a Comprehensive Service Contract for the equipment requested? | |||
i. Is suitable accommodation available to install the equipment (building and facilities) and is the structure suitable to carry the additional mass? This to be confirmed by the Engineering Services Manager, in writing. | |||
j. Are there engineering services available to operate the equipment and has the availability of the service been confirmed in writing by the Engineering Services Manager, for instance (as for above scenario – Checklist 5)? | |||
k. Can this equipment be standardised for the reasons previously stated above? | |||
l. Statistics of patients to be treated with the equipment requested. | |||
m. Are there no other procedures available to treat the patients? | |||
n. Will not having the equipment in any way compromise patient care or safety? | |||
o. Are there proper specifications from the Health Technology Unit available, or is suitable equipment available from an approved period tender? State the tender and the item numbers. | |||
Checklist 5: '''Request to purchase additional equipment similar to equipment already in use''' | |||
a. Inventory of similar equipment available in the Institution. | |||
b. Inventory of how many pieces of the type of equipment are functional. | |||
c. Inventory of how many pieces of the type of equipment are not functional and the reason therefore. | |||
d. Is this equipment in line with the norms of service delivery – level of care - of the institution? | |||
e. Reason for requesting the additional equipment backed up with patient statistics. Has the function or status of the Institution changed? | |||
f. Does the institution have sufficient funds to purchase the equipment? | |||
g. Does the institution have a budget, sufficient funds to pay for a comprehensive service contract for the equipment requested? | |||
h. Can this equipment be standardised for the reasons previously stated? | |||
i. Are there no other procedures available to treat the patients? | |||
j. Will not having the equipment in any way compromise patient care or safety? Are there proper specifications available from the Health Technology Unit or is suitable equipment available from an approved period tender. State the tender and item numbers? | |||
==Procurement== | |||
===Introduction=== | |||
Procurement, from a healthcare service perspective, is probably the most important activity within the medical equipment life-cycle. If the right (competent) people are driving a transparent, criteria-driven (not interest-driven) process, it is likely that the healthcare system will avail itself of the technology that is most appropriate to the intended application, while addressing issues around effectiveness, cost-of-ownership, institutional fit, technology maturity and compatibility, user competence, maintenance and so on. In many countries, procurement is being placed in the hands of generic supply-chain management personnel with little specialised knowledge of medical equipment specifically, and health technologies in general. It is therefore essential to ensure that a supporting environment is created to minimise the possibility of sub-optimal procurement outcomes. | |||
Procurement (for capital equipment as opposed to consumables) should be seen as a process with two phases: one that commences with planning and needs assessment and ends with commissioning, and the other that extends over its operational lifetime and ensures that the equipment is provided with the necessary accessories, consumables, maintenance support, and so on. | |||
There are four reasons for procuring equipment, each of which provides a different goal which will dictate when to acquire equipment. These can be placed in the following order of priority (see '''''Box 8''''' below). Within each of the four categories shown, priorities will have to be set, and these can be based on appropriate indicators. | |||
Box 8: '''Example of valid reasons and order of priority for purchasing of equipment''' | |||
{| class="wikitable" | |||
|1. '''To cover depreciation of equipment.''' | |||
|Equipment is replaced as it reaches the end of its life and is taken out of service. This is necessary in order for the level of healthcare that is currently delivered to be sustained. [Note: This means that the size of the existing equipment stock remains the same, and does not imply an expansion of the health service.] | |||
|- | |||
|2. '''To obtain additional equipment items which are missing from the basic standard requirements.''' | |||
|Additional equipment may be required in order to provide a basic standard level of care. [Note: Missing items are identified by comparing the Equipment Inventory with the Standard Equipment List for the facility.] | |||
|- | |||
|3. '''To obtain additional equipment items beyond the basic standard.''' | |||
|This is done in order to upgrade the level of health service provided by the hospital. For example, new equipment may be needed to provide a new service, build a new special unit, or increase the level of care offered. | |||
|- | |||
|4. '''To obtain additional equipment items outside the facility’s own plans.''' | |||
|This will only be applicable if the additional items have been called for by directives from the national or provincial ministry, and cannot be stopped/refused for political reasons, such as “out of the ordinary”, high profile, or political projects. | |||
|} | |||
Of course, procurement is not a self-contained, isolated process but links up with many other equipment-related processes; these are shown in '''''Box 9''''' below. | |||
Box 9: '''Planning tools that assist in deciding on procurement''' | |||
{| class="wikitable" | |||
|'''Planning tools''' | |||
|'''How they help''' | |||
|- | |||
| colspan="2" |'''When replacing items:''' | |||
|- | |||
|Replacement policy | |||
|Establishing and implementing this tool is much more likely to ensure that the necessary regular planned replacement of equipment takes place. | |||
|- | |||
|Equipment inventory and maintenance record system | |||
|These tools support identification of the need for replacement equipment at any time. For easy reference, estimated lifetimes for equipment could be entered into the inventory and could then prompt one when to purchase. The natural life of equipment is shortened by harsh environment, over-use, unskilled handling, neglect of maintenance and damage. Equipment malfunction and downtime also increase with the age of the equipment. Accordingly, cost-effectiveness decreases with age. | |||
|- | |||
|Replacement and condemnation criteria | |||
|These assist with judging when equipment has reached the end of its life, and therefore with identifying when equipment needs replacing. | |||
|- | |||
|Core equipment expenditure plan | |||
|Some <ins>of the </ins>equipment will have to be replaced every year, so it makes sense to spread budgeting for replacement over time. By allocating some money each year, one can avoid facing a large replacement bill later on. This tool should spread these costs over the long term. Equipment replacement needs can be estimated each year by assuming that each piece of equipment has an average lifetime of 10 years [1] - '''this means that on average 10% of equipment stock needs to be replaced each year.''' | |||
|- | |||
|Disposal policy and disposal procedures | |||
|When equipment is replaced, these tools will assist with the disposal of the old device or system. There may be government regulations regarding disposal that will provide additional information. | |||
|- | |||
|Stock<ins>-</ins> control system | |||
|Replacement equipment-related supplies should be purchased after an up-to-date stock take. Accurate stock control systems help with planning and ordering. | |||
|- | |||
| colspan="2" |'''When buying additional new items:''' | |||
|- | |||
|Equipment development plan, and annual purchase plan | |||
|New equipment should be purchased according to <ins>an </ins>annual purchase plan (drawn each year from <ins>a </ins>long-term equipment development plan). This is based on an up-to-date inventory and Standard Equipment List. Accurate inventory record-keeping helps with planning and ordering. | |||
|- | |||
|Package of inputs | |||
|Planning in advance for what will be needed after the purchase is every bit as important as the purchase itself. Thus, one must ensure that the package of inputs required to keep equipment functioning through its life is procured. | |||
|- | |||
|Core equipment financing plan, and annual budget | |||
|Capital expenditure can only take place once sufficient funding sources have been identified. The long-term Core Equipment Financing Plan and Annual Budget should allocate known and possible sources of funds against elements of planned expenditure. | |||
|} | |||
Additional items of equipment may need to be procured to accompany/complement the original item, if it is to function properly in certain environments. For example: | |||
*a voltage stabiliser (surge suppressor plus filter) - this offers protection against power supply fluctuations, but does not protect against power cuts. It monitors the power supply, removes surges and spikes, and maintains a continuously regulated alternating current output to the item; | |||
*an uninterruptible power supply - this offers protection against blackouts and power cuts of limited duration; | |||
*an air-conditioning unit; and | |||
*a water filter or treatment plant. | |||
===Preparation of specifications=== | |||
Specifications have to be drawn up for every device that is planned to be purchased. Standardised specifications need to be drawn up for commonly used devices which can then be modified (where necessary) at institutional level. | |||
General terms and conditions should also be part of specifications<ins>,</ins> including stipulations like the local availability of essential spare parts, and the presence of a registered sole agent for the specific brand. | |||
Specifications should be functional specifications and drawn up based on features available in at least a few brands of the device commonly available in South Africa. Before making a selection the following may be considered: | |||
*demonstration of devices by supplier; | |||
*trial use of the device in a facility; | |||
*visit to a facility where the device is available; | |||
*verbal presentation on device made by a supplier of the device; and | |||
*communication with existing users of the device both locally and abroad. | |||
A suggested format for specifications is as follows: | |||
*name of equipment; | |||
*Function; | |||
*essential features; | |||
*essential components; | |||
*additional components; | |||
*power supply; | |||
*additional requirements; and | |||
*training – user training, maintenance training. | |||
In order to eliminate the possibility of outdated specifications being used, all specifications should have a validity date on the document. Specifications that are not valid or have expired should not be used. Copies of the latest specifications should be obtained from the appropriate HTM unit. | |||
A sample specification (that for an infant incubator) is given in '''Annex I'''. For some equipment, such as sophisticated or imported items, or equipment which is new in the system, it may be necessary to specify the following item lines: | |||
*'''Site preparation details''' – supplier should provide technical instructions and details so that this work can be planned, either in-house or by contracting out. | |||
*'''Installation''' – assistance may be needed. | |||
*'''Commissioning''' – assistance may again be required. | |||
*'''Acceptance''' – the responsibilities of both the purchaser and supplier with respect to testing and/or acceptance of the goods must be clearly detailed. | |||
*'''Training''' of '''both''' users and technicians – help must be obtained if required. | |||
*'''Maintenance contract''' (an important part of after-sales support) – help must be requested if it is required. It will be necessary to agree and stipulate the duration<ins>,</ins> and whether it should extend beyond the warranty period, the cost and whether it includes the price of labour and spare parts, and the responsibilities of the owner and supplier. | |||
There are a number of technical and environmental factors that need to be taken into account. For example: | |||
*If the area has an unstable power supply, is the supplier able to offer technical solutions (such as voltage stabilisers, <ins>an </ins>uninterruptible power supply)? | |||
*Will the geographical location (such as height above sea-level) affect the operation of equipment (such as motors, pressure vessels)? If so, can the manufacturer adjust the item<ins>’s</ins> specific needs? | |||
*Extremes of temperature, humidity, and dust may adversely affect equipment operation, and may require solutions such as air-conditioning, silica gel, polymerised coatings for printed circuit boards, and filters. | |||
This information can be included within the generic equipment specifications. However, since much of the information is common to many pieces of equipment, some health service providers have found it simpler to develop a separate summary ''Technical and Environmental Data Sheet'', which can be referred to in the purchase documents. This data sheet can be distributed to all suppliers, interested parties, trade delegations and other relevant bodies. Such a data sheet can be provided regardless of the length of specification or the procurement method used, ensuring that all parties are kept informed of prevailing national conditions which could affect the operation of equipment. | |||
The following details should be included in a Technical and Environmental Data Sheet: | |||
*Electricity supply – mains or other supply, voltage and frequency values and fluctuations. | |||
*Water supply – mains or other supply, quality and pressure. | |||
*Environment: height above sea-level; mean temperature and fluctuations; humidity; dust level; vermin problems, etc. | |||
*Manufacturing quality – international or local standards required. | |||
*Language required – main and secondary. | |||
*Technology level required – manual, electro-mechanical or micro-processor controlled. | |||
===Evaluation and comparison process=== | |||
The process of evaluation and comparison can often be time consuming. However, it is important to ensure that decisions for awarding contracts are not made simply on the basis of which items are the cheapest. During evaluation the items should be assessed against the requirements specified in the purchase document. The most common way to evaluate offers is to use an elimination process, where some offers are rejected at each stage. Offers should be judged against the following criteria: | |||
*Compliance with requirements in the purchase document. | |||
*Technical nature of the offer (part of the product selection criteria). | |||
*Financial nature of the offer (part of the product selection criteria). | |||
*Supplier qualification criteria. | |||
By doing this, decisions are based on best value for money for the whole life-cycle cost, rather than simply being based on the item’s purchase price. | |||
The process of evaluation and comparison must be fair and thorough. To achieve this, the process must follow a defined pattern to ensure all bids/quotes are dealt with in exactly the same way. | |||
The evaluation process for tenders is similar to that for quotes, although the tender process is normally a far more comprehensive task and is also regulated by law. The tools for evaluation can be the same, but the amount of information required is usually much less with quotation methods. Obviously, if only three quotes are requested for a simple small order, the evaluation process should not take much time. The following evaluation steps are used, and some bids/quotes are rejected at the end of each step. | |||
'''Step 1: Checking for compliance''' | |||
The first step when evaluating offers is to determine which, if any, are not compliant with the technical, commercial, and other specifications in the purchase document. The procurement unit should be able to do this. It involves a more detailed examination of the offer to determine the compliance by the bidder with the requirements specified in the purchase document. This is known in tender processes as the '''substantial responsiveness''' of the bid. This process is more formal and comprehensive for tenders than for quotations. | |||
A substantially responsive bid is one which: | |||
*conforms to all the terms and conditions; this means that the supplier has responded to all parts of the schedule of requirements, has filled in all the boxes, and is able to supply all the parts required; and | |||
*also establishes the bidder’s qualifications to supply and deliver the products within the delivery schedule; for example, the supplier: | |||
- has enclosed signed audited accounts, signed company declarations on health, safety, and environmental activities, certificates of quality manufacturing, and a letter of authorisation from the manufacturer, and | |||
- has nominated a representative in the country (if this was one of the compliance criteria). | |||
All non-substantial bids will be rejected as non-responsive and should be excluded from further in-depth evaluation. | |||
In practice it is found that most bids contain reservations from one or more of the detailed requirements. If these are just minor adjustments and do not represent a substantial deviation from expressed interests, one can still conclude that the bid is substantially responsive and therefore should be included. However, the reservations for closer clarification should be listed. | |||
To assist in this examination one could ask for other clarifications from the bidder. One may also wish to inspect the quality methods and production methods stated in their documents. This request and response should be in writing. The bidders, however, are not allowed to make any changes to the substance or price of their offers. After evaluating the offers for compliance, some offers are discarded as the suppliers fail. The remaining offers can go forward to be compared in terms of technical performance (Step 3). | |||
'''Step 2: Preparing the evaluation information''' | |||
The procurement unit should collate and compile the information from all the responsive bids into evaluation information sheets. '''''Box 10''''' gives an example of the sort of data that needs to be included. | |||
Box 10: '''Evaluation information sheets''' | |||
{| class="wikitable" | |||
|Evaluation Information Sheets present an easy way to compare different bids/quotes. For ease of comparison, it is best to allocate one page per subject matter (technical product details, price, etc.) and compare the offers side-by-side. Areas where each offer deviates from the equipment specification should be highlighted. Typical evaluation information sheets would include the following evaluation criteria: | |||
''Technical product data:'' | |||
*The international/regional standards that the equipment adheres to. | |||
*Whether the operational method used is new, well tried, or old. | |||
*The level of sophistication of the technology and the operating technique. | |||
*Which functions and measurements do not meet the specifications in the purchase document. | |||
*Whether any quality assurance certificates apply to the equipment. | |||
''Accessories, consumables, spare parts:'' | |||
*A list and the cost of accessories included in the offer. | |||
*A list and the cost of consumables included in the offer. | |||
*A list and the cost of spare parts included in the offer. | |||
*The length of time spare parts will be available and their location. | |||
''After-sales service:'' | |||
*Cost of after-sales support. | |||
*Guarantee/warranty periods. | |||
*The details of the after-sales support that can be provided. | |||
''Price information:'' | |||
*The price. | |||
*Delivery arrangements | |||
*Costs to install, commission, and train. | |||
*The life-cycle costs. | |||
Note: this is an example and not a comprehensive list. The information chosen to include will depend upon the complexity of the equipment purchased – less technical detail is needed for simple equipment or recurrent supplies. | |||
|} | |||
Ideally the evaluation information sheets should allow side-by-side comparison of the offers. For tenders there are more responses and the orders tend to be more complex, so more paperwork is required to obtain a true comparison. Usually there is too much information to be compiled in a single sheet, so it may be better to use: | |||
*one sheet for collating purely technical information about the product; | |||
*one for information on accessories, consumables, and spare parts; | |||
*one for after-sales service; and | |||
*one for price information. | |||
The procurement unit should present the evaluation information sheets to the procurement/tender committee (for tenders and high-value quotes) or the Procurement Manager (for low-value quotes). They should be accompanied by an explanation of the requirements, main objectives, and any binding constraints. Then the technical evaluation process (Step 3) can commence. | |||
'''Step 3: Carrying out the technical evaluation''' | |||
The procurement/tender committee should base its decision on advice from members of the group with professional knowledge relevant to the offer. If appropriate, it should also seek advice from representatives from the relevant user department and HTM team (for low-value quotes, the Procurement Manager should also seek advice from these end-users). | |||
The committee should consider the product selection criteria, as set out in the purchase document. It is essential that the information provided by the supplier is related only to the equipment specification and the selection criteria listed in the purchase document – any additional information intended to sway the evaluation can be seen as corruption; hence the importance of detailing requirements adequately in the purchase document. The better the purchase document, the easier the evaluation process will be. '''''Box 11''''' lists a few points to remember, relating to the technical aspects of the offer. | |||
Box 11: '''Summary technical assessment''' | |||
{| class="wikitable" | |||
| | |||
*If a detailed specification was issued, then every detail of the offer must be closely checked to make sure it conforms to the original specification. | |||
*If only a brief specification was issued, then the checks should be limited to ensuring the equipment or service offered meets the requirements. | |||
*Any modifications or alternatives offered must be assessed individually, and decisions made whether any one is more cost-effective than another. | |||
*The technical assessment includes suitability, safety, ability to commission and train, after-sales support, warranty arrangements and more – do not simply assess technical data concerning the equipment. | |||
|} | |||
For both quotations and tenders it may be necessary to ask the supplier to clarify any ambiguities or uncertainties. Alternatively, a shortlist of suppliers can be drawn up and those included can be asked to demonstrate their equipment, as part of the technical evaluation (this is unlikely to be necessary for very simple equipment, and may be impossible for many overseas suppliers). | |||
After the technical evaluation, some offers are discarded if they fail to meet the requirements. The remaining offers can go forward to be compared in terms of financial performance. | |||
===Issues to consider when choosing equipment=== | |||
Choosing equipment is not easy, due to the wide range of products available. External influences also play a part. For instance, external support agencies may impose their own conditions regarding suppliers, which may result in inappropriate equipment being supplied or procured. The acquisition policy should clearly specify the “good selection criteria” to employ. All equipment should: | |||
*be appropriate to its intended target setting; | |||
*be of assured quality and safety; | |||
*be affordable and cost-effective; | |||
*be easily used and maintained; and | |||
*conform to the existing policies, plans and guidelines. | |||
These criteria, unpacked in '''''Checklist 6''''' below, should be used during the procurement process when evaluating and adjudicating between different offers from suppliers. | |||
Checklist 6: '''Example of good selection criteria for equipment purchasing''' | |||
{| class="wikitable" | |||
|'''Indicators of appropriateness''' | |||
|'''Criteria''' | |||
|- | |||
|Appropriate to setting | |||
|Equipment should be: | |||
*suitable for the level of facility and service provided; | |||
*acceptable to staff and patients; | |||
*suitable for operator skills available; | |||
*suitable for the local maintenance support capabilities; | |||
*compatible with existing equipment and consumable supplies; | |||
*compatible with existing utilities and energy supplies; | |||
*suited to the local climate, geography and conditions; and | |||
*able to be run economically with local resources. | |||
|- | |||
|Assured quality and safety | |||
|Equipment should be: | |||
*of sufficient quality to meet institutional requirements and last a reasonable length of time; | |||
*made of materials that are durable and hard-wearing; | |||
*made from material that can be easily cleaned, disinfected, or sterilised without rusting; | |||
*manufactured to meet internationally recognised safety and performance standards; | |||
*suitably packaged and labelled so that it is not damaged in transit or during storage; and | |||
*provided by reputable, reliable, licensed manufacturers, or registered suppliers. | |||
|- | |||
|Affordable and cost-effective | |||
|Equipment should be: | |||
*available at a price that is cost-effective. Quality and cost often go together (for example, the cheaper option may be of poor quality and ultimately prove to be a false economy); | |||
*affordable in terms of costs for freight, insurance, import tax, etc.; | |||
*affordable in terms of installation, commissioning, and training of staff to use and maintain them; | |||
*affordable to operate (for example, cover the costs of consumables, accessories, spare parts and fuel over its lifetime); | |||
*affordable to maintain and service; | |||
*affordable to dispose of safely; | |||
*affordable in terms of the procurement process (for example the cost of a procurement agent or foreign exchange); and | |||
*affordable in terms of staffing costs (for example, costs of any additional staff or specialisation training required). | |||
<br /> | |||
|- | |||
|Ease of use and maintenance | |||
|Equipment should be chosen | |||
*for which the necessary skills in terms of operating, cleaning and maintenance are available; | |||
*for which instructions and manuals are available in a suitable language; | |||
*for which staff training is offered by the supplier; | |||
*for which local after-sales support is available with real technical skills; | |||
*which offers the possibility of additional technical assistance through service contracts; | |||
*which comes with a warranty/guarantee, covering a reasonable length of time, for which the terms are understood <s>the terms</s> (for example, does it cover parts, labour, travel, refunds or replacements?); | |||
*which offers a supply route for equipment-related supplies (for example, consumables, accessories, spare parts); and | |||
*which offers assured availability of these supplies for a reasonable period (up to 10 years). | |||
|- | |||
|Conforms to existing policies, plans and guidelines | |||
|Equipment should be chosen: | |||
*according to the purchasing and donations policy; | |||
*according to the standardisation policy; | |||
*according to the technology level described in the Standard Equipment Lists and Generic Equipment Specifications; | |||
*which is deemed to be suitable, having studied available literature and compared products; and | |||
*which is deemed to be suitable, having received feedback regarding previous purchases. | |||
|} | |||
It may also be appropriate to have a set of criteria to evaluate equipment suppliers, both current and past, as per '''''Checklist 7'''''. | |||
Checklist 7: '''Suggested criteria for evaluating current and past suppliers''' | |||
{| class="wikitable" | |||
|'''Issue''' | |||
|'''Criteria''' | |||
|- | |||
|Participation record | |||
| | |||
*Has the supplier accepted an award of bid/quotation and subsequently failed to deliver? | |||
*Has the supplier attempted to alter or withdraw bids after submission? | |||
*Has the supplier promised after-sales support, then failed to provide it? | |||
|- | |||
|Response to enquiry | |||
| | |||
*Has the supplier adequately responded to all enquiries and within a reasonable period of time? | |||
*Did the supplier provide regular updates on the status of outstanding orders? | |||
|- | |||
|Delivery time (for consumables) | |||
| | |||
*What was the supplier’s average promised lead time? | |||
*What was the actual lead time for the last round of procurement? | |||
*What additional costs were incurred due to late shipments (if applicable)? | |||
|- | |||
|Adherence to delivery instructions | |||
| | |||
*Did the shipments arrive under proper shipping conditions? | |||
*Did the supplier send full shipments as requested, or were there partial shipments? | |||
|- | |||
|Provisions of documents | |||
| | |||
*Were advance copies of documents provided according to contract terms? | |||
*Did shipments arrive with all required documents correctly filled out and signed? | |||
*Were adequate site preparation instructions provided? | |||
*Were adequate training materials provided? | |||
*If required documents were omitted, how did the supplier correct the problem? | |||
|- | |||
|Packing and labelling (mainly for consumables) | |||
| | |||
*Was labelling complete and adequate for proper use? Was it in the correct language? | |||
*Did the supplier ship the correct package size? Were the correct contents and quantities in each package? | |||
*Did external packaging protect the product from damage during transport in country? | |||
|- | |||
|Product shelf life (for consumables) | |||
| | |||
*Did the products comply with contractual terms for remaining shelf life? If not, how many were shipped with shelf life less than specified? | |||
*Did the supplier replace items that did not have acceptable remaining shelf life or allow the return for credit or exchange of goods nearing their expiration date? | |||
|- | |||
|Compliance with contract financial terms | |||
| | |||
*Did all invoices comply with contract pricing terms? | |||
*Were all shipments correctly insured and shipped, according to financial terms in the contract? | |||
*Were there any problems obtaining compensation or reimbursement for lost or damaged goods? | |||
*Were there any problems obtaining compensation or reimbursement for poor services (such as commissioning and training)? | |||
|- | |||
|Quality of products and services | |||
| | |||
*Have complaints been received concerning product quality for this supplier? | |||
*Did the supplier cooperate in making samples available and paying for any quality-control tests performed by quality-assurance agencies? | |||
*Have any complaints been received concerning the quality of services provided by this supplier (such as installation, commissioning, and initial training)? | |||
*Have any complaints been received concerning the quality of after-sales support from this supplier? | |||
|} | |||
''Adapted from'': Management Sciences for Health, 2002, “Managing drug supply”, MSH, Boston, USA. | |||
===Donations=== | |||
Donations are a special case of procurement, and great care needs to be exercised in managing the procurement process associated with donated equipment. The World Health Organization (WHO)[1] and other bodies have drafted guidelines to facilitate this process. | |||
==Receipt, testing, installation & commissioning== | |||
===Pre-installation work / site preparation=== | |||
The site at which the device is to be installed has to be adequately prepared, more so in the case of large, sophisticated devices like x-rays, autoclaves. This has to be coordinated with logistics of the device supply so that the site is ready when the device is delivered at the institution. The associated utilities like appropriate power supply, water, compressed air and the like, as well as other appropriate requirements like radiation protection should also be taken into consideration. In situations where extensive site preparation is necessary, like in x-rays or CT scans, site works should be included as part of the acquisition process to ensure comprehensive and coordinated site preparation. | |||
Pre-installation work involves: | |||
*preparing the site ready for equipment when it arrives; | |||
*organising any lifting equipment; | |||
*organising any warehouse (storage) space; | |||
*confirming installation and commissioning details; and | |||
*confirming training details. | |||
In some cases, the pre-installation work required is minimal; in others it requires considerable labour and finance. As a general guide, site preparation is the work required to ensure that the room or space where the equipment will be installed is suitable. It often requires the provision of new service supply connections (for electricity, water, drainage, gas, waste) and may require some construction work. Site preparation tasks can include: | |||
*disposing of the existing obsolete item (disconnection, removal, cannibalising for parts, transport, decontamination and disposal); | |||
*extending pipelines and supply connections to the site, from the existing service installations; | |||
*upgrading the type of supply, such as increasing the voltage, or the pipeline diameter; | |||
*providing new surfaces, such as laying concrete, or providing new worktops; and | |||
*creating the correct installation site – for example, digging trenches, building a transformer house or a compressor housing. | |||
In considering where to position equipment, the following types of questions should be asked: | |||
*Is there sufficient access to the room/space? (Door sizes and elevator capacity are very important for x-ray and other large machines.) | |||
*Is the room/space large enough? | |||
*Is the position and layout of the room/space suitable? | |||
*Are the required work surfaces and service supply points available? | |||
*Is the environment adequate for the purpose? (For example, is it air-conditioned? Dust-free? Away from running water?) | |||
If new buildings or extensions are being constructed, different relevant departments and groups need to work closely together to design the rooms and plan the service supplies. Planners, users, architects, service engineers, and equipment engineers need to be consulted. | |||
Site preparation can be carried out by: | |||
*in-house staff (for example, the facility HTM team or a central/regional HTM team); | |||
*maintenance staff from other national agencies (for example, electricians from the Ministry of Public Works); | |||
*a contractor who has been hired (for example, a private company or an NGO partner); and | |||
*the suppliers or their representative. | |||
When planning and budgeting for the equipment, site-preparation costs should have been estimated for inclusion in the budget. However, as soon as the order is placed, the HTM working group / procurement unit should provide the supplier with details of the proposed equipment site and services, and officially request the necessary site preparation instructions. | |||
Once these are received the HTM manager can plan the work, quantify the needs and costs for materials and contractors, and apply for a budget allocation. He or she should then oversee the work, and ensure it is undertaken before the equipment arrives. | |||
'''''Flowchart 8''''' below shows the common site preparation steps that may be required, depending on the type of equipment purchased. By the time the goods start to arrive, the site should be ready to receive them. | |||
FLOWCHART 8: '''Common site preparation steps''' | |||
[[File:Flowchart 8 - Common site preparation steps.PNG|thumb|none|720x720px]] | |||
===An overview of the acceptance process=== | |||
Each health facility should have an official '''acceptance process''' for equipment that arrives on site (a simpler process is used when equipment-related supplies arrive on their own). During the acceptance process, the following should be established: | |||
the complete order has arrived; | |||
*installation, commissioning, and initial training has taken place; | |||
*the equipment is mechanically and electrically safe for users and patients and is functioning properly; and | |||
* the equipment is entered into the health facility inventory. | |||
A simple way to carry out these activities is to fill in a standard Acceptance Test Logsheet. This form is specially designed to make checking easier and to help to avoid mistakes. It is an important document since it is the first record to be placed in the equipment file and provides all relevant details of the start of the equipment’s life at the health facility, and commences the service history of the equipment. The Acceptance Test Logsheet has sections that cover all the components of the acceptance process, including: | |||
*delivery/receipt of the equipment on site; | |||
*unpacking and checking for damage and for the complete order; | |||
*assembly; | |||
*installation; | |||
*commissioning and safety testing; | |||
*official acceptance; | |||
*initial training; | |||
*registration – entering stocks into stores and onto records; and | |||
*handover. | |||
Each of these sections in the logsheet needs to be completed and signed off to indicate that the activity has been successfully completed. Once the logsheet has been fully completed, it is signed off to certify that the equipment and services are satisfactory. Only then should payment be made. | |||
If there are problems with goods or services, the Acceptance Test Logsheet should '''not''' be signed; instead <s>write</s> a fault report on the equipment’s shortcomings should be logged, outlining the problems encountered and advising that payment be withheld until the problems have been addressed. | |||
The equipment is not normally put into routine use until the complaints have been resolved, the logsheet finally signed off, and the payments made. The acceptance process is straightforward for common low-complexity items of equipment that are simple to use. Installation, commissioning, and initial training are not major activities and can happen all at once – e.g. for a mobile examination lamp: | |||
*Installation involves using a test meter to check the electricity supply of the socket outlet, and then simply plugging in the lamp. | |||
*Commissioning involves using a test meter to check the electrical safety of the lamp so that it will not give the operator an electric shock. | |||
*Initial training involves ensuring the operator knows where the on/off switch is, how to handle the light bulb, and how to alter the angle of the head without pulling the lamp over. | |||
====Commissioning==== | |||
Commissioning usually, but not always, takes place straight after installation. The technical and safety aspects of the equipment should already have been specified and considered during the selection process. However, it is also essential to carry out performance and safety tests on each piece of equipment. Such tests validate that each piece of equipment is safe and is capable of performing its intended function. Performance and safety tests should be carried out regardless of whether equipment is purchased, donated, leased or borrowed by the health facility. The commissioning team and any visiting installers are responsible for ensuring these tests take place. The steps in the commissioning process are shown in '''''Flowchart 9'''''. | |||
FLOWCHART 9: Key steps in the commissioning process[[File:Flowchart 9 - Key steps in the commissioning process.PNG|thumb|none|720x720px]] | |||
Once the equipment has passed its safety, calibration, and function tests, the commissioning team is in a position to: | |||
*officially accept that the equipment has been received in a satisfactory condition, and | |||
*officially accept the equipment as the institution/department’s property. | |||
This could trigger the payment for the goods only. Payment for services can only occur when the training is finished, if it was part of the purchase contract. If the equipment has not passed the tests, negotiations would be commenced with the supplier and complaints procedures initiated. The equipment should '''not be''' accepted or used until these issues have been resolved. Once the equipment is accepted, staff can be trained in its operation and maintenance. | |||
===Registration and handover=== | |||
Once equipment and supplies have been officially accepted, they can be registered in various health facility records and systems, process them, and stored or used as appropriate. The commissioning team is responsible for ensuring that all these activities take place. | |||
====Entering new equipment orders into health facility records==== | |||
All equipment and equipment-related supplies need to be entered into the health facility’s records and systems. The most common records and systems are the: | |||
*'''Equipment Inventory''' (manual or computerised). The HTM manager should enter new major pieces of equipment onto the Equipment Inventory. The type of information recorded should identify the particular piece of equipment, its manufacturer and location. The HTM manager can gather this information from the Acceptance Test Logsheet and the Register of New Stocks form. In addition, the HTM manager should allocate a unique '''inventory code number''' to each piece of equipment and ensure the equipment is labelled/marked with this code number. | |||
*'''Equipment File''' (manual or computerised). This acts as a service history for a particular piece of equipment. The HTM manager should open a new Equipment File for each piece of equipment, and label the file with the equipment’s inventory code number. The type of information recorded in this file at the acceptance stage should include details of the manufacturer/supplier and purchase contract terms. Much of this information will be contained in the completed Acceptance Test Logsheet, which should be the first document placed in this file with supplementary data as required. Subsequent records placed in the Equipment File are of any maintenance work carried out so that the file becomes the equipment’s service history. | |||
*'''Planned preventive maintenance (PPM) programme.''' The HTM manager should register equipment for any PPM carried out by maintenance staff (if it is taking place), and enter it onto their '''PPM timetable''' so that it gets attention at regular intervals. On handover of the equipment to the user, the HTM manager should liaise with the head of the user department about registering the equipment for any user PPM [BvR1] taking place, and entering it onto their user PPM timetable [see '''Section 10.2''' for further discussion on maintenance]. | |||
*'''Equipment card'''. This is a piece of card or laminated sheet that is permanently kept with the equipment. It can provide users with a summary of the equipment care instructions and a summary service history, such as dates when routine inspections, testing, and servicing took place. | |||
*'''Register of New Stocks form.''' This provides the Stores Controller with all the information required for entering each new piece of equipment and its supplies into the stores stock control system. The commissioning team should (partially) complete this form by gathering information from the available contract, packing lists, or invoice, according to a standard format. The type of information should identify the manufacturer/supplier’s order codes, description, and batch sizes of the equipment, consumables, accessories, and spare parts. The Stores Controller finishes completing the form by allocating and recording each item’s unique stores code. The Stores Controller provides copies of the finalised forms to the user departments and HTM team so they can order the correct replacement items in future. The HTM manager keeps this form in the relevant Equipment File. | |||
*'''Registering warranties.''' The guarantee or warranty of the equipment (if applicable) needs to be registered. The date of commencement and the period of the warranty which have been agreed with the supplier must be entered into all relevant procurement, finance, and maintenance records. | |||
*'''''Note'':''' ''Once the equipment has been checked and is confirmed as safe and ready for use, it is sensible to highlight this fact. Use a simple strip of tape placed across the main controls of the apparatus to clearly show that it has been tested. It is preferable to use printed warning notice tape, but other types could be used.'' | |||
====Storing manuals==== | |||
Operator and service manuals should be supplied with the equipment, according to the purchase document. It is important to make sure that manuals are kept in a safe place, and are not lost by staff. It is also important to make them widely available for use among users and maintenance staff. This can be done by: | |||
*Storing the original copies in a safe place, such as the clinical engineering workshop library, the main stores office, or the HTM service library. | |||
*Making two photocopies of all operator manuals received, and giving one set to the head of the relevant user department, and the other to the HTM team or workshop responsible for the equipment’s maintenance. | |||
*Making one photocopy of the service manuals received, and giving it to the HTM team or workshop responsible for the equipment’s maintenance. | |||
*Asking for manuals or their content in other formats, such as CD-Rom, video, DVD. | |||
*Scanning the printed documents into a computer to convert them into electronic copies, and making them easily available to maintenance staff at many locations. | |||
*Recording in the Equipment File how the manuals were distributed. This helps with monitoring and updating the manuals in future. | |||
==Training== | |||
===Introduction=== | |||
After successful installation and commissioning, the users and maintainers need training on the type of equipment and the model purchased. This training can take place straight after commissioning, with the installation team acting as the trainers. However, the training team often involves different people, such as those with clinical or training skills. In this case, the training may take place sometime later when the training team has assembled. | |||
Ideally, the initial training occurs straight after commissioning. After the training, the equipment can be handed over to the user department for regular use. However, if there is a delay before training can take place, one may have to consider whether to hand over the equipment before training staff. We recognise that there will be pressure to do this as all staff want to start using new equipment as soon as possible. | |||
However, this should only be done if: | |||
*the equipment is a type that has been used before; | |||
*the staff are familiar with the equipment; and | |||
*experienced staff members are in charge of its use, until the remaining staff can be trained. | |||
'''Note:''' Anyone using or working on equipment without proper training or authority whose actions result in an accident is likely to be found negligent. | |||
Depending on the complexity of the equipment and the staff’s previous experience of it, initial training can include: | |||
*good practice when handling the equipment; | |||
*basic “dos and don’ts”; | |||
*how to operate the equipment (along with familiarisation with the symbols and markings on the machine); | |||
*the correct application of the equipment; | |||
*care, cleaning, and decontamination; | |||
*safety procedures; | |||
*planned preventive maintenance (PPM) for users; and | |||
*PPM and repair for maintainers. | |||
The level and nature of training provided depends on whether the equipment is: | |||
*'''A standard make and model.''' If staff are familiar with the equipment, in-house staff could train new users and provide refresher training for other staff. The training sub-group should produce suitable and necessary training resources and handouts for the trainees, using the operator and service manuals, and any videos available. | |||
*'''A new make or model.''' If the equipment is unfamiliar, training should be carried out by the supplier or their representative, or by a central training team with knowledge of the equipment. The training sub-group should observe the training session, obtain copies of any overheads or handouts used, and compile its own training pack for future training. | |||
Sometimes in-depth training is given only to very few staff members who, at a later stage, will train the rest of the users. In this case, a training timetable is needed to ensure the ongoing training occurs. | |||
Note: | |||
*One should ensure that the chosen trainees turn up for the training sessions, and maintain records of the training that individual staff members have received. | |||
*A library of the training resources developed should be established. | |||
*The representatives of both the commissioning team and visiting training team should sign the Acceptance Test Logsheet, to avoid later disputes. | |||
===Correct application=== | |||
Staff may feel confident about how to operate equipment, but it is imperative that they also know the correct “application” for the equipment. Staff need to be able to apply their taught (clinical) procedures correctly, and to employ the correct methods of application so that equipment is used to its fullest capacity. | |||
Staff will need to be trained in order to fully appreciate when and how to use equipment. They will need to know: | |||
*when different features will be employed for different patients or uses the range of assistance a machine can offer them; | |||
*how to alter the relationship between the machine and the patient, or sample, for different purposes; and | |||
*the different procedures to pursue for different disorders or treatments. | |||
They will also need to understand the safety precautions they must take. Traditionally, colleges offering basic training for health are responsible for teaching clinical procedures. Thus, they must have access to the necessary equipment for this purpose, both in their teaching rooms and at suitable clinical locations such as hospitals. | |||
It is important to note that training is an ongoing endeavour. Staff come and go, and even staff who have received initial training on an item of equipment may need refresher training. It is therefore useful to have a mechanism that will trigger training interventions. '''''Flowchart 10''''' suggests appropriate triggers. | |||
Flowchart 10: '''Example of prompts showing that training is required''' | |||
[[File:Flowchart 10 - Example of prompts showing that training is required.PNG|thumb|none|720x720px]] | |||
When the device is removed from service, professional users should know how to clean it and to organise decontamination. | |||
===Special case: for home-based care or patient-operated devices=== | |||
Professional users, clinical supervisors and prescribers need to make sure that training for end-users enables them to use a medical device/equipment safely and effectively, and to perform routine maintenance as appropriate for the medical device/equipment. For example, end-users of ambulatory infusion pumps should be aware of how the medical device works, including special features such as bolus delivery, and the risks of siphoning if a syringe is removed from a driver. It is also essential that end-users are provided with information contained in manufacturer’s instructions and that it is explained and, where necessary, expanded upon. Where possible, user organisations should provide the same standards of equipment and training for end-users as they do for staff. | |||
== Operation== | |||
===Introduction=== | |||
“Operation” of equipment means using the correct physical methods to get the equipment to work. In order to do this successfully, the user needs to know: | |||
*the specific operating characteristics of the equipment; | |||
*the operational procedures that make the machine work; | |||
*how to use its various functions; | |||
*how to make it perform its customary cycles and routines; and | |||
*how to change the bulb, paper roll, batteries, etc. | |||
The equipment manufacturer’s user manual is often the best source of this information. Other sources include: | |||
*written resources from staff training sessions; | |||
*experienced colleagues; and | |||
*a wide range of reference material. | |||
'''''Box 12''''' provides some examples of general strategies when operating equipment | |||
Box 12. '''General strategies when operating equipment (for users)''' | |||
{| class="wikitable" | |||
| | |||
*Make sure you have the authority and knowledge to operate equipment before you start; | |||
*Refer to the manufacturer’s operating manual for correct advice. | |||
*'''Always''' follow the safety procedures specific to the machine. | |||
*Only connect a machine to an electrical socket outlet which has been checked by the electrician for proper grounding, and only use the correct plug for the socket (for example, '''never''' put a two-pin plug into a three-pin socket). | |||
*Do '''not''' start equipment that requires water for its operation without checking that the water supply is available. | |||
*Use equipment/machinery only with the correct type and quantities of water, oil, fuel, etc. | |||
*Always handle accessories carefully as these are the most easily damaged component of equipment. | |||
*Always use the correct consumables without wasting them. | |||
*Always respond to alarms, and check what is causing them. | |||
*Ensure that the relevant waste pipeline is not blocked, before allowing equipment to discharge its contents into the waste water/sewage system or special container for hazardous material. | |||
*If the electricity power supply is cut off, switch off all electrical equipment to protect it from electrical surges which may occur when the power is restored; once power is returned, ensure that vital equipment (such as refrigerators) is switched back on. | |||
*Ensure that any mains-powered equipment which will be required to work off batteries during a power failure (defibrillator, vital signs monitor, ICU ventilator, etc.) is always plugged into a live socket and '''left switched on''' so that its batteries are continuously charged. | |||
*When oxygen and other gases are no longer required, '''turn off''' the supply so that gas is not discharged into the atmosphere and wasted. | |||
<br /> | |||
|} | |||
Each type of equipment has specific operating instructions. When operating equipment, it is also essential to follow good strategies for using consumable materials. | |||
Equipment users should only operate equipment for which they are suitably qualified. Clinical meetings, committee meetings, departmental meetings, or specifically organised training sessions can all be used to provide application training (as appropriate). | |||
For specialised devices e.g. autoclave, x-rays, the right type of personnel are required to operate these devices. Certification may be a requirement for some specialised devices. Regular monitoring has to be carried out for certain specialised devices (e.g. radiation safety monitoring for x-ray machines). User manuals should be provided for all devices to enable users to operate all devices safely and effectively, as well as for trouble-shooting for simple problems encountered with devices. | |||
===Care and cleaning=== | |||
To maximise the life of equipment, it is necessary that equipment users and maintainers know how to look after the equipment and clean it. Users must care for and clean equipment regularly, to a given timetable. It is beneficial to do this because: | |||
*it is easier to see faults (such as damaged suction pump tubing) when the equipment is clean; | |||
*it prolongs the life of equipment (for example, protecting electronic parts from damage by dust, protecting metal from corrosion by liquids or chemicals, protecting rubber seals from degradation by greases); | |||
*it protects the operator and patient from infections (from microscope eyepieces, for example); and | |||
*it improves the performance of equipment (clean probes for ultrasound, clean seals on fridge doors, etc.). | |||
The best information regarding the care and cleaning of equipment is usually contained in the manufacturer’s user manual and/or service manual. In addition, staff should have written resources from their training sessions and, in some cases, posters which provide the guidance and experience of their colleagues. | |||
===Equipment-related infection control=== | |||
Internationally, the term “infection control” has come to mean control of a wide range of practices, processes and procedures in the clinical work of the health facility as a whole. Proper infection control (relating to equipment) can be achieved by making decisions about a number of different issues: | |||
*decontamination, through appropriate use of cleaning, disinfection, and sterilisation methods, as well as monitoring sterility; | |||
*linen handling; and | |||
*ensuring the workplace is clean. | |||
Information and advice must be sought from the relevant national body that is responsible for infection control, since national policies should have been established to ensure that risks are reduced. It is suggested that the central HTM working group, or its smaller sub-group, the infection control committee, should be responsible for establishing policies and guidelines to prevent contamination through exposure to blood, body fluids, body parts, and infectious agents. | |||
===Staff accountability=== | |||
Finally, the issue of staff accountability should be addressed. '''''Checklist 8''''' includes checks for different role-players as part of a systemic approach. | |||
Checklist 8: '''Strategies for making staff more accountable''' | |||
{| class="wikitable" | |||
|'''Type of Staff''' | |||
|'''Strategies''' | |||
|- | |||
|Heads of section | |||
|<nowiki>- given overall responsibility for the equipment in their section, according to their inventory and undertake regular inventory checks to look for missing equipment and accessories;</nowiki> | |||
- made responsible for conforming to the local security regulations for the facility and its site; and | |||
- ensure their staff have sufficient skills to operate and care for equipment correctly and safely, and help them to access appropriate training or reference materials. | |||
|- | |||
|Operators-in-charge | |||
|<nowiki>- uring every shift, made responsible for undertaking functional checks on the equipment as part of user planned preventive maintenance (PPM) schedules.</nowiki> | |||
|- | |||
|Equipment users | |||
|<nowiki>- made responsible for the state of the equipment, accessories and consumables they handle and use;</nowiki> | |||
- take personal responsibility for ensuring they operate equipment correctly, and that they know the correct operating techniques and applications; | |||
- take personal responsibility for using the correct consumables in a non-wasteful way; | |||
- take personal responsibility for ensuring they operate equipment safely, and knowing the proper safety procedures; | |||
- made responsible for the daily care and cleaning of the equipment they use with the correct cleaning chemicals; | |||
- made responsible for monitoring that equipment is functioning properly and is providing the type of results expected, and reporting any faults immediately to the HTM team through their head of section; | |||
- ensure they have been specifically trained to undertake these tasks, and if they require further skill-development put in a request to their head of section; and | |||
- take responsibility for conforming to the local security regulations for the facility and its site. | |||
|- | |||
|purchasing and supplies officers | |||
|<nowiki>- made responsible for purchasing the correct consumables and cleaning chemicals.</nowiki> | |||
|- | |||
|Stores controllers | |||
|<nowiki>- made responsible for keeping track of stocks of consumables and materials in the various stores.</nowiki> | |||
|- | |||
|Health management teams | |||
|<nowiki>- develop a suitable working environment where managers are seen to be present, and performing well themselves;</nowiki> | |||
- consider positive strategies with bonuses and rewards for good behaviour with equipment, as incentives for making staff more responsible and accountable; and | |||
- consider disciplinary mechanisms so that staff are charged for intentional loss of, or damage to equipment and accessories, to make them more accountable for their actions. | |||
|} | |||
==Maintenance, risk-management and safety== | |||
===Introduction=== | |||
An effective medical equipment maintenance programme consists of adequate planning, management and implementation. Planning considers the financial, physical and human resources required to adequately implement the maintenance activities. Once the programme has been defined, financial, personnel and operational aspects are continually examined and managed to ensure the programme continues uninterrupted and improves as necessary. Ultimately, proper implementation of the programme is key to ensuring optimal equipment functionality. '''''Box 13''''' lists the some of the responsibilities and tools typically associated with a medical equipment maintenance system. | |||
Box 13: '''Summary of registers, ledgers and files used for maintenance and who keeps them''' | |||
{| class="wikitable" | |||
|Equipment users and managers | |||
|<nowiki>- </nowiki>'''Work request/job forms''' used for recording the details of the request for maintenance support and also used by maintainers to record the work done. | |||
- '''User department maintenance files''' to keep track of the requests made and their progress. | |||
- '''User PPM schedules''' for each type of equipment, '''PPM timetables''' and '''wall calendars.''' | |||
- '''Equipment cards''' attached to/kept with all pieces of equipment, showing user PPM and care instructions and service due dates. | |||
|- | |||
|Maintainers | |||
|<nowiki>- </nowiki>'''Work request/job forms''' submitted by equipment users and used by the maintainers for recording the details of the work undertaken. | |||
- '<nowiki/>''Personal record books'''[1]'<nowiki/>''''' to keep track of special problems and successes with work, which help to identify skill development needs. | |||
- '''PPM schedules''' for each type of equipment. | |||
- '''Equipment cards''' attached to/kept with all pieces of equipment used by the maintainers for recording dates when routine inspections, testing, and servicing took place, and are next due. | |||
|- | |||
|HTM manager (and possibly a clerk) | |||
|<nowiki>- </nowiki>'''Inventories''' of equipment, furniture, buildings, tools, etc. | |||
- '''PPM timetables''' and '''wall calendars''' showing when PPM was undertaken on different devices. | |||
- '''Job pending files''' and '''job completed files''' kept by the HTM team to keep track of the requests received and their progress. | |||
- A '''contractors’ record book/file''' to record which contractor undertook which job, and their performance. | |||
- '''Equipment files''' and '''section files''' which create service histories. | |||
- '''Statistics forms''' and '''statistics folders''' for keeping various statistical records for analysis and for compiling written reports for management. | |||
|- | |||
|HTM team’s stores-person | |||
|<nowiki>- A </nowiki>'''tools ledger''' in order to keep a record of: the tools owned, which staff members were allocated which tools, and the return of the tools at the end of the day. | |||
- '''Stock cards''' used by the HTM team’s stores-person to record and keep track of the different items stocked in the storeroom. | |||
- A '''stock control ledger''' kept by the HTM team in order to monitor the quantities of parts and materials used and kept in stock. | |||
|} | |||
*Simple repairs should be carried out by users. Other repairs should be carried out by clinical engineering personnel. Specialised or complex repairs should be carried out by suppliers. | |||
*Planned preventive maintenance should be carried out to minimise down-time of devices (e.g. to replace x-ray tube at time period recommended by manufacturer rather than when tube fails). | |||
*Calibration and testing should be carried out at regular intervals, as recommended by the manufacturer, by users or clinical engineering personnel – depending on the complexity of the device. | |||
*Records of maintenance and repairs of devices should be documented in a maintenance log, while a plant register is to be maintained for sophisticated or specialised devices. | |||
A maintenance strategy includes procedures for inspection, as well as preventive and corrective maintenance. Performance inspections ensure that equipment is operating correctly, safety inspections ensure the equipment is safe for both patients and operators, and preventive maintenance (PM) aims to extend the life of the equipment and reduce failure rates. Additionally, some hidden problems may be discovered during a scheduled inspection. However, performing inspections of equipment only ensures that the device is in good operating condition at the time of inspection and cannot eliminate the possibility of failure during future use; the nature of most electrical and mechanical components is that they can potentially fail at any time. Corrective maintenance (CM) restores the function of a failed device and allows it to be put back into service. | |||
===Risk-based maintenance strategy=== | |||
A risk-based maintenance strategy prioritises maintenance resources toward assets that carry the most risk if they were to fail. It is a methodology for determining the most economical use of maintenance resources. This is done so that the maintenance effort across a facility is optimised to minimise the total risk of failure. | |||
A risk-based maintenance strategy is based on two main phases: | |||
*Risk assessment. | |||
*Maintenance planning based on the risk. | |||
The maintenance frequency and type are prioritised based on the risk of failure. Assets that have a greater risk and consequence of failure are maintained and monitored more frequently. Assets that carry a lower risk are subjected to a less stringent maintenance programme. By this process, the total risk of failure is minimised across the facility in the most economical way. | |||
Risk is adopted as an index for clarifying priority in risk maintenance[BvR1] technologies. That risk can be given as the product of the probability of failure occurring to the inspected area and the consequence of failure to the surroundings. In other words, risk is the expected value of the degree of impact. '''''Figure 1''''' shows the concept of distribution of risk held by devices in a system. | |||
Figure 1: '''Distribution of risk for medical devices''' | |||
[[File:Distribution of risk for medical devices.png|thumb|none|720x720px]] | |||
A to S on the horizontal axis are the devices in a system and the vertical axis is the relative risk each device holds. The graph shows that 80% of the total risk of the system is concentrated in just 20% of the devices. In that case, it would be irrational to inspect all devices with the same level of priority. It is thus important to identify the 20% of devices and raise their priority level in the inspection programme. That is the basic concept of risk maintenance technology. | |||
A framework for determining risk in the health industry should be implemented in the following manner: | |||
FIGURE 2: Risk-based maintenance framework | |||
[[File:Risk-based maintenance framework.png|thumb|none|720x720px]] | |||
====Data collection - equipment groups==== | |||
For each risk that has been identified data about the risk needs to be collected. In the equipment scenario it is always advisable to group equipment with the same nature and/or function, instead of focusing on individual items. Information per equipment group will include information about the risk, its general consequences and the general methods used to mitigate and predict the risk. | |||
In a model developed as part of the IUSS project the main equipment groups identified are shown in Table 3 (also shown are other factors to be discussed later). | |||
Table 3: '''Equipment groupings and related risk and maintenance factors''' | |||
{| class="wikitable" | |||
|'''GRP CODE''' | |||
|'''GRP DESCRIPTION NDoH''' | |||
|'''Defined risk''' | |||
|'''Total risk Factor (%)''' | |||
|'''% Maint. of repl. value''' | |||
|- | |||
|CAUCLE | |||
|Auxiliary Cleaning/Sterilising Equipment | |||
|B-Medium | |||
|56% | |||
|3.5% | |||
|- | |||
|CAUOTH | |||
|Auxiliary Other Specialised Equipment | |||
|B-Medium | |||
|52% | |||
|5.5% | |||
|- | |||
|CAUSPE | |||
|Auxiliary Special Medical furniture | |||
|B-Medium | |||
|68% | |||
|3.0% | |||
|- | |||
|CAUMED | |||
|Auxiliary General Medical furniture | |||
|C-Low | |||
|20% | |||
|3.0% | |||
|- | |||
|CAUTES | |||
|Auxiliary Test Equipment | |||
|B-Medium | |||
|74% | |||
|5.5% | |||
|- | |||
|CAUFUR | |||
|Auxiliary General Administrative Furniture | |||
|C-Low | |||
|18% | |||
|2.5% | |||
|- | |||
|CAUAPP | |||
|Auxiliary General Appliances | |||
|C-Low | |||
|20% | |||
|3.0% | |||
|- | |||
|CAUSOF | |||
|Auxiliary Software and Communication Systems | |||
|B-Medium | |||
|74% | |||
|12.0% | |||
|- | |||
|CBDAUD | |||
|Diagnostic Audiology | |||
|B-Medium | |||
|72% | |||
|5.5% | |||
|- | |||
|CBDFLE | |||
|Diagnostic Flexible Endoscopes | |||
|B-Medium | |||
|74% | |||
|5.5% | |||
|- | |||
|CBDMIC | |||
|Diagnostic Microscopes | |||
|B-Medium | |||
|74% | |||
|5.5% | |||
|- | |||
|CBDPAT | |||
|Diagnostic Pathology Devices | |||
|B-Medium | |||
|66% | |||
|5.5% | |||
|- | |||
|CBDRIG | |||
|Diagnostic Rigid Scopes | |||
|B-Medium | |||
|62% | |||
|5.5% | |||
|- | |||
|CBDSCA | |||
|Diagnostic Scales | |||
|C-Low | |||
|48% | |||
|5.5% | |||
|- | |||
|CBDSUR | |||
|Diagnostic Surgical Lights | |||
|B-Medium | |||
|74% | |||
|3.0% | |||
|- | |||
|CBDVID | |||
|Diagnostic Video/Data Devices | |||
|B-Medium | |||
|50% | |||
|5.5% | |||
|- | |||
|CBDOPT | |||
|Diagnostic Ophthalmology | |||
|B-Medium | |||
|72% | |||
|5.5% | |||
|- | |||
|CBLANA | |||
|Life Support Anaesthesia Units | |||
|A-High | |||
|100% | |||
|5.5% | |||
|- | |||
|CBLBAL | |||
|Life Support IABP | |||
|A-High | |||
|100% | |||
|5.5% | |||
|- | |||
|CBLDEF | |||
|Life Support Cardioversion Devices | |||
|A-High | |||
|88% | |||
|5.5% | |||
|- | |||
|CBLHRT | |||
|Life Support Heart-Lung Bypass Units | |||
|A-High | |||
|96% | |||
|5.5% | |||
|- | |||
|CBLVEN | |||
|Life Support Ventilators | |||
|A-High | |||
|88% | |||
|5.5% | |||
|- | |||
|CBMCAP | |||
|Monitoring Capnography | |||
|B-Medium | |||
|72% | |||
|5.5% | |||
|- | |||
|CBMCEN | |||
|Monitoring Central Stations | |||
|B-Medium | |||
|60% | |||
|3.0% | |||
|- | |||
|CBMECG | |||
|Monitoring ECG | |||
|B-Medium | |||
|60% | |||
|5.5% | |||
|- | |||
|CBMMUL | |||
|Monitoring Multi-Parameter Monitors | |||
|B-Medium | |||
|66% | |||
|5.5% | |||
|- | |||
|CBMOTH | |||
|Monitoring Other Patient Parameters | |||
|B-Medium | |||
|64% | |||
|5.5% | |||
|- | |||
|CBMPRE | |||
|Monitoring Pressures | |||
|B-Medium | |||
|60% | |||
|5.5% | |||
|- | |||
|CBMSAT | |||
|Monitoring Saturation | |||
|B-Medium | |||
|60% | |||
|5.5% | |||
|- | |||
|CBMTEM | |||
|Monitoring Temperature | |||
|B-Medium | |||
|60% | |||
|5.5% | |||
|- | |||
|CBTBEU | |||
|Therapeutic Neuromuscular Stimulators | |||
|A-High | |||
|76% | |||
|5.5% | |||
|- | |||
|CBTBRE | |||
|Therapeutic Breast Pumps | |||
|C-Low | |||
|40% | |||
|5.5% | |||
|- | |||
|CBTHEM | |||
|Therapeutic Hemofiltration Units | |||
|A-High | |||
|76% | |||
|5.5% | |||
|- | |||
|CBTINC | |||
|Therapeutic Infant Warming and Phototherapy | |||
|A-High | |||
|88% | |||
|5.5% | |||
|- | |||
|CBTINF | |||
|Therapeutic Infusion Devices | |||
|B-Medium | |||
|64% | |||
|5.5% | |||
|- | |||
|CBTINS | |||
|Therapeutic Inspired Gas Conditioning Devices | |||
|B-Medium | |||
|64% | |||
|5.5% | |||
|- | |||
|CBTLAZ | |||
|Therapeutic Lasers | |||
|A-High | |||
|82% | |||
|5.5% | |||
|- | |||
|CBTWAR | |||
|Therapeutic Warmers Patient/Blood/Solution | |||
|B-Medium | |||
|74%. | |||
|5.5% | |||
|- | |||
|CBTDEN | |||
|Therapeutic Dental Equipment | |||
|B-Medium | |||
|60% | |||
|5.5% | |||
|- | |||
|CBTPHY | |||
|Therapeutic Physiotherapy Equipment | |||
|B-Medium | |||
|64% | |||
|5.5% | |||
|- | |||
|CINELE | |||
|Instruments Electric | |||
|B-Medium | |||
|74% | |||
|5.5% | |||
|- | |||
|CINMAN | |||
|Instruments Manual | |||
|B-Medium | |||
|62% | |||
|2.0% | |||
|- | |||
|CINPHN | |||
|Instruments Pneumatic | |||
|B-Medium | |||
|74% | |||
|5.5% | |||
|- | |||
|CINHOL | |||
|Instruments Hollow ware & General Stainless ware | |||
|C-Low | |||
|20% | |||
|2.0% | |||
|- | |||
|CRDAUX | |||
|Radiology Digital Auxiliary Equipment | |||
|B-Medium | |||
|72% | |||
|9.0% | |||
|- | |||
|CRDBDE | |||
|Radiology Digital Bone Densitometers | |||
|A-High | |||
|80% | |||
|5.5% | |||
|- | |||
|CRDCAT | |||
|Radiology Digital Computed Tomography | |||
|A-High | |||
|84% | |||
|9.0% | |||
|- | |||
|CRDDEN | |||
|Radiology Digital Radiographic Units Dental | |||
|A-High | |||
|80% | |||
|9.0% | |||
|- | |||
|CRDMRI | |||
|Radiology Digital Magnetic Resonance Imaging | |||
|A-High | |||
|84% | |||
|9.0% | |||
|- | |||
|CRDRFS | |||
|Radiology Digital Radiographic/Fluoroscopic Systems | |||
|A-High | |||
|84% | |||
|9.0% | |||
|- | |||
|CRDRSD | |||
|Radiology Digital Radiographic Systems | |||
|A-High | |||
|80% | |||
|9.0% | |||
|- | |||
|CRDULT | |||
|Radiology Digital Ultrasonic Scanning Systems | |||
|A-High | |||
|76% | |||
|6.0% | |||
|- | |||
|CRDNUC | |||
|Radiology Nuclear Medicine Equipment | |||
|A-High | |||
|84% | |||
|9.0% | |||
|} | |||
====Risk evaluation - factors impacting on risk==== | |||
At the risk-evaluation stage, both the probability of the risk and the consequence of the risk are quantified in the context of the equipment group under consideration. For this specific model, more emphasis has been placed on risk factors impacting directly on the maintenance function. | |||
'''<u>Volume</u>''' | |||
Availability of alternative devices to perform the same required function | |||
'''Score Intensity''' | |||
'''5''' - Very low volume (only one per hospital). | |||
'''4''' - Low volume. | |||
'''3''' - Medium (loan units available). | |||
'''2''' - Moderate (some duplication exist). | |||
'''1''' - High volume (high numbers per unit). | |||
'''<u>Function</u>''' | |||
Equipment function has a direct impact on the risk associated with the equipment: | |||
'''Score Function'''<blockquote> | |||
Therapeutic equipment | |||
</blockquote>'''5''' – Life-support. | |||
'''4.5 ''' - Surgical and intensive care. | |||
'''4''' - Physical therapy and treatment.<blockquote> | |||
Diagnostic equipment | |||
</blockquote>'''3.5''' - Surgical and intensive care monitoring. | |||
'''3''' - Additional physiological monitoring and diagnostic. <blockquote> | |||
Analytical equipment | |||
</blockquote>'''2.5 ''' - Analytical laboratory. | |||
'''2 ''' - Laboratory accessories. | |||
'''1.5 ''' - Computer and related.<blockquote>Miscellaneous equipment</blockquote>'''1 ''' - Patient Related Furniture and Other. | |||
'''<u>Physical risk</u>''' | |||
The risk associated with equipment failure and/or misuse measured by the impact on patient and/or staff injury | |||
'''Score Risk''' | |||
'''5''' - Patient death. | |||
'''4''' - Patient or operator injury. | |||
'''3''' - Inappropriate therapy or misdiagnosis. | |||
'''2''' - Operational risk. | |||
'''1''' - No significant risk. | |||
'''<u>Maintenance intensity</u>''' | |||
Maintenance intensity in the operational environment also impacts on the risk model due to the specific requirements placed on scarce resources such as technical staff and financial constraints. | |||
'''Score Intensity''' | |||
'''5 - ''' Extensive and costly with highly skilled technical staff involved. | |||
'''4''' - Moderate skilled technical resources required but still costly. | |||
'''3''' - Average skilled technical resources required with low cost associated. | |||
'''2''' - Low technical skill required with low cost. | |||
'''1''' - Minimal maintenance required. | |||
'''<u>Technology intensity</u>''' | |||
This is the risk associated with the requirements placed on the operator due to the nature and technology level of the equipment. Qualified operators and trained staff remain a scarce resource in the medical device industry. | |||
'''Score Intensity''' | |||
'''5''' - Operator-certified and specialised training required. | |||
'''4''' - Specialist nursing training required. | |||
'''3''' - Average training required. | |||
'''2''' - Very low skill level required. | |||
'''1''' - No training required. | |||
<br /> | |||
====Risk ranking and risk classes==== | |||
With the risk evaluation complete, the probability and consequence are combined to determine the total risk. This total risk is ranked against pre-determined levels of risk. As a result, the risk is either acceptable or unacceptable. | |||
Equipment groups can therefore be divided into three major classes according to the risk evaluation outcome: | |||
'''<u>High-risk equipment – risk score of 75% and higher</u>''' | |||
*''Risk is not acceptable and preventive maintenance and condition monitoring is highly advised.'' | |||
*''This category includes all life-support devices, key resuscitation devices, and other devices whose failure or misuse is reasonably likely to result in serious injury to patients or staff.'' | |||
*''High impact on hospital operational activities.'' | |||
*''Low volume of items increase<ins>s</ins> risk.'' | |||
*''Device history could increase or reduce risk.'' | |||
'''<u>Medium-risk equipment – risk score of 50% to 75%</u>''' | |||
*''Risk is not acceptable and preventive maintenance and/or condition monitoring advised.'' | |||
*''This category includes devices whose misuse, failure, or absence would have significant impact on patient care, but would not likely cause immediate serious injury.'' | |||
*''Medium impact on hospital operational activities.'' | |||
*''Low volume of devices raises risk.'' | |||
*''Device history could increase or reduce risk.'' | |||
'''<u>Low-risk equipment – risk score of 50% and lower</u>''' | |||
*''Risk is acceptable and very low level of preventive maintenance – if any maintenance is advised.'' | |||
*''This category includes devices whose failure or misuse is unlikely to have serious consequences.'' | |||
*''Very low impact on hospital operational activities.'' | |||
*''High volume of devices reduce<ins>s</ins> risk.'' | |||
====Inspection and preventive maintenance planning==== | |||
Maintenance planning should always start with a basic approach <s>were</s> <ins>where </ins>only staff and maintenance material are required, and further evolve to a more advanced approach where the specific asset and method required for the asset are addressed. This also forms the basis for the maintenance excellence hierarchy. The preventative maintenance planning is based on the following diagram: | |||
FIGURE 3: The maintenance planning process | |||
[[File:The maintenance planning process.png|thumb|none|720x720px]] | |||
====Equipment supported==== | |||
The starting point for any advanced maintenance approach is to know exactly what is being maintained. The model therefore makes use of Standard Equipment List(s) obtained from NDoH. The Standard Equipment List (SEL) per level of care is a list of the medical equipment in the various nursing departments and provides detail per room level. The SELs for the various levels of care are combined in the model and standardised according to Universal Medical Device Nomenclature System™ (UMDNS). The combined equipment list is grouped according to the various equipment groups and the risk model applied. | |||
Final quantities of equipment determined by number of facilities to achieve a theoretical equipment list per province/district. This theoretical HT Asset Register can now be further manipulated to accept changes made to quantities of specific unique items. | |||
====Method - maintenance procedures and intervals==== | |||
Maintenance procedures and intervals are determined by the manufacturer and/or legal requirements. There are international minimum accepted norms available to determine not only the service/inspection interval ('''''Table 4''''') but also the minimum procedure to be performed (''NB with Table 4: All other types: once a year''). | |||
Table 4: '''Maintenance intervals for different equipment types''' | |||
{| class="wikitable" | |||
|'''Equipment type''' | |||
|'''Interval p.a.''' | |||
|'''Equipment type''' | |||
|'''Interval p.a.''' | |||
|- | |||
|Anaesthesia Unit Vaporisers | |||
|2 | |||
|Infusion Devices | |||
|1 | |||
|- | |||
|Anaesthesia Unit Ventilators | |||
|2 | |||
|Intra-Aortic Balloon Pumps | |||
|2 | |||
|- | |||
|Anaesthesia Units | |||
|2 | |||
|Isolated Power Systems | |||
|2 | |||
|- | |||
|Apnoea Monitors | |||
|1 | |||
|Laparoscopic Insufflators | |||
|1 | |||
|- | |||
|Argon-Enhanced Coagulation Units | |||
|1 | |||
|Mammography Units | |||
|2 | |||
|- | |||
|Argon Surgical Lasers | |||
|2 | |||
|Medical Gas/Vacuum Systems | |||
|1 | |||
|- | |||
|Aspirators, Emergency and Tracheal | |||
|2 | |||
|Mini C-arms | |||
|2 | |||
|- | |||
|Automated External Defibrillators | |||
|2 | |||
|Mobile C-arms | |||
|2 | |||
|- | |||
|Autotransfusion Units | |||
|2 | |||
|Mobile High-Efficiency-Filter Air Cleaners | |||
|4 | |||
|- | |||
|Beds, Electric | |||
|1 | |||
|Mobile x-ray Units | |||
|2 | |||
|- | |||
|Blood Pressure Monitors, Electronic Indirect | |||
|1 | |||
|Nd:YAG Surgical Lasers | |||
|2 | |||
|- | |||
|Blood Pressure Monitors, Invasive | |||
|1 | |||
|Oxygen-Air Proportioners | |||
|1 | |||
|- | |||
|Blood/Solution Warmers | |||
|1 | |||
|Oxygen Analysers | |||
|1 | |||
|- | |||
|Capnometers and Multiple Medical Gas Monitors | |||
|1 | |||
|Oxygen Concentrators | |||
|4 | |||
|- | |||
|Carbon Dioxide Surgical Lasers | |||
|2 | |||
|Pacemakers, External Invasive | |||
|1 | |||
|- | |||
|Cardiac Resuscitators | |||
|2 | |||
|Pacemakers, External Non-invasive | |||
|2 | |||
|- | |||
|Centrifuges | |||
|1 | |||
|Peritoneal Dialysis Units | |||
|2 | |||
|- | |||
|Circulating-Fluid Pumps | |||
|1 | |||
|Phototherapy Units | |||
|1 | |||
|- | |||
|Circumcision Clamps | |||
|1 | |||
|Physical Therapy Ultrasound Units | |||
|1 | |||
|- | |||
|Conductive Furniture and Floors | |||
|12 | |||
|Pneumatic Tourniquets | |||
|2 | |||
|- | |||
|Critical Care Ventilators | |||
|2 | |||
|Portable Ventilators | |||
|2 | |||
|- | |||
|Cryosurgical Units | |||
|1 | |||
|Pressure Transducers | |||
|2 | |||
|- | |||
|Defibrillators | |||
|2 | |||
|Pulmonary Resuscitators, Gas-Powered | |||
|1 | |||
|- | |||
|ECG Monitors | |||
|1 | |||
|Pulmonary Resuscitators, Manual | |||
|1 | |||
|- | |||
|Electrical Receptacles | |||
|1 | |||
|Pulse Oximeters | |||
|1 | |||
|- | |||
|Electrocardiographs | |||
|1 | |||
|Radiant Warmers | |||
|1 | |||
|- | |||
|Electrosurgical Units | |||
|2 | |||
|Radiographic Units, General Purpose | |||
|2 | |||
|- | |||
|Fetal Monitors | |||
|1 | |||
|Radiographic/Fluoroscopic Units, General-Purpose | |||
|2 | |||
|- | |||
|Frequency-Doubled Nd:YAG Surgical Lasers | |||
|2 | |||
|Smoke Evacuators | |||
|1 | |||
|- | |||
|Heart-Lung Bypass Units | |||
|³4 | |||
|Sphygmomanometers | |||
|1 | |||
|- | |||
|Heated Humidifiers | |||
|1 | |||
|Suction Regulators, Tracheal<sup>*</sup> | |||
|2 | |||
|- | |||
|Hemodialysis Units | |||
|4 | |||
|Temperature Monitors | |||
|1 | |||
|- | |||
|Ho:YAG Surgical Lasers | |||
|2 | |||
|Traction Units | |||
|1 | |||
|- | |||
|Hypo/Hyperthermia Units | |||
|1 | |||
|Transcutaneous O<sub>2</sub>/CO<sub>2</sub> Monitors | |||
|1 | |||
|- | |||
|Infant Incubators | |||
|1 | |||
|Ultrasound Scanners | |||
|1 | |||
|} | |||
====Materials – service required items==== | |||
Material required for preventive maintenance is very device- and manufacturer-specific and cannot be generalised or averaged to be used in the model. The majority of the maintenance and repair workload is simple tasks requiring common spare parts. Therefore, try to remember that more than 80% of all spare parts needed (in theory), represent less than 20% of the overall spare parts cost. In other words, only 20% of your maintenance and repair tasks can eat up 80% of your spare parts budget, because the equipment is complex and expensive. | |||
====Staff – human resource requirement==== | |||
The maintenance and repair requirements of healthcare facilities are more demanding than those of most other facilities. The high intensity of use, long operating hours (24-hours per day for most primary care hospitals) and high-technology specialised equipment all lead to a relatively high maintenance workload usually fulfilled by a combination of internal staff and external service providers. | |||
There are a number of statistical models to determine the ideal staff level but most fail owing to the complexity of the different variables: | |||
*Technology level of hospital. | |||
*Availability of internal maintenance specialisation. | |||
*Internal vs outsourced maintenance combination. | |||
*Cost constraints. | |||
*Etc. | |||
To get a better estimation of staff levels as well as the impact of outsourced maintenance, this model takes a number of specific factors related to staff into consideration: | |||
====Maintenance intensity of specific equipment==== | |||
The combined theoretical equipment list contains a total of 768 unique equipment descriptions. Each equipment type has been graded according to the service requirement assigned by the service procedure and interval, as follows: | |||
Table 5: '''Grading of equipment service requirements''' | |||
{| class="wikitable" | |||
|'''Level''' | |||
|'''Major Service''' | |||
|'''Minor Service''' | |||
|'''Verification''' | |||
|'''Predictive Inspection''' | |||
|'''Reactive Maintenance''' | |||
|- | |||
|'''1''' | |||
|'''XXX''' | |||
|'''XXX''' | |||
|'''XXX''' | |||
|'''XXX''' | |||
|'''XXX''' | |||
|- | |||
|'''2''' | |||
| | |||
|'''XXX''' | |||
|'''XXX''' | |||
|'''XXX''' | |||
|'''XXX''' | |||
|- | |||
|'''3''' | |||
| | |||
| | |||
|'''XXX''' | |||
|'''XXX''' | |||
|'''XXX''' | |||
|- | |||
|'''4''' | |||
| | |||
| | |||
| | |||
|'''XXX''' | |||
|'''XXX''' | |||
|- | |||
|'''5''' | |||
| | |||
| | |||
| | |||
| | |||
|'''XXX''' | |||
|} | |||
*'''Major service''' | |||
A service that normally requires the replacement of parts as well as consumable service items. | |||
*'''Minor service''' | |||
A service that normally only requires the replacement of consumable service items. | |||
*'''Verification''' | |||
The verification of equipment performance to a standard in the form of a performance analyser and/or simulator. | |||
*'''Predictive inspection''' | |||
The inspection of equipment to a set list. No tools and/or verification equipment required. | |||
*'''Reactive maintenance''' | |||
Only reactive work performed on equipment breakdown and/or failure. | |||
The specific level of maintenance is always determined by the highest service requirement and may not always include the lower service requirements. A device that requires a major service but no minor services or verification will therefore be graded as Level 1 maintenance intensity, although not all services are required. | |||
====Maintenance trade classification==== | |||
The specific staff skill required as well as the skill level is assigned to the service requirement of every unique equipment description. | |||
*'''Electronic high''' | |||
Electronically qualified person with specialisation on specific equipment. | |||
*'''Electronic medium''' | |||
Electronically qualified person without specialisation on specific equipment. | |||
*'''Electronic low''' | |||
Person with electronic background but no formal tertiary qualification. | |||
*'''Mechanical high''' | |||
Mechanically qualified person with specialisation on specific equipment. | |||
*'''Mechanical medium''' | |||
Mechanically qualified person without specialisation on specific equipment. | |||
*'''Mechanical low''' | |||
Person with mechanical background but no formal tertiary qualification. | |||
====Maintenance labour time requirement==== | |||
An average of labour time per unique equipment description is calculated based on equipment group risk (maintenance intensity) and assigns the following values per service type: | |||
Table 6: '''Labour hours per service type''' | |||
{| class="wikitable" | |||
|'''Labour hours''' | |||
| colspan="5" |'''Maintenance intensity risk score''' | |||
|- | |||
|'''Service type''' | |||
|'''1''' | |||
|'''2''' | |||
|'''3''' | |||
|'''4''' | |||
|'''5''' | |||
|- | |||
|'''Major service''' | |||
|'''1''' | |||
|'''1.5''' | |||
|'''2.5''' | |||
|'''3.5''' | |||
|'''4.5''' | |||
|- | |||
|'''Minor service''' | |||
|'''0.5''' | |||
|'''0.75''' | |||
|'''1.25''' | |||
|'''1.75''' | |||
|'''2.25''' | |||
|- | |||
|'''Verification''' | |||
|'''0.5''' | |||
|'''0.5''' | |||
|'''0.5''' | |||
|'''0.5''' | |||
|'''0.5''' | |||
|- | |||
|'''Predictive maintenance''' | |||
|'''0.25''' | |||
|'''0.25''' | |||
|'''0.25''' | |||
|'''0.25''' | |||
|'''0.25''' | |||
|} | |||
The only equipment group that does not form part of the above table is radiology equipment, where equipment verification has been extended to an average of two hours per verification. | |||
====Outsourcing of maintenance activities==== | |||
Many equipment types require very specialised training and tools for preventative as well as reactive maintenance. This high specialisation requirement on the maintenance often leads to the outsourcing of maintenance. Outsourcing could occur per equipment group or per unique device (manufacturer specific). | |||
This model incorporates three different categories that can be user selected per unique equipment type: | |||
*Outsourced | |||
All maintenance activities related to the specific equipment are performed by outsourced service providers. | |||
*Combined | |||
The major and minor services as well as reactive maintenance are performed by outsourced service providers and verification and predictive maintenance are performed by internal staff. As a norm, the ratio of outsourced labour to internal labour is set at 70% to 30%. The ratio can be changed to accommodate various scenarios. | |||
*Internal | |||
All maintenance activities performed by internal staff with the exception of some reactive maintenance outsourced (unique cases). | |||
The various types of outsourcing contracts include: | |||
*'''Full coverage contracts''' shall be considered contracts which include both unscheduled (emergency repair) and scheduled (preventive/interval) maintenance. This type of coverage will involve the least amount of work for the operator and the Health Technology/Clinical Engineering Department, as it will be up to the vendor to complete all maintenance of the equipment. The time invested in the management of the contract will involve the most effort by the Health Technology Department as the billing will have to be monitored and the contract will need to be overseen to ensure that all provisions of the contract(s) are being carried out in a timely manner. | |||
*'''Unscheduled maintenance only''' (emergency repair) contracts are desirable where the ability to perform the scheduled maintenance programme exists within the hospital. Each item of equipment should receive a minimum of one (1) preventive maintenance inspection per year by the internal staff. | |||
*'''Scheduled maintenance only''' (preventive/interval maintenance inspection) contracts shall be considered cost-effective where the need for expensive specialised test equipment is needed and is not owned by the hospital, and/or where the reliability of the equipment is such that the unscheduled downtime of the equipment does not warrant the need for full coverage. Cost-effectiveness shall be a major part in this decision. | |||
*'''Parts only''' contracts shall be considered where the expertise to perform the work required is contained within the facility but the cost of parts is expensive. The decision to acquire this type of contract shall be supported by documentation to the cost of parts and the capability of the Biomedical Engineering staff. | |||
*Variations of and combinations of the aforementioned are another possibility. Where documentation is present, it is possible to decide the most cost-effective method of coverage for each piece of equipment. The extent of coverage and any portion of the workload which can be performed by the in-house staff shall be prime consideration to the exact type of coverage required. | |||
====Implementation of preventative maintenance plan==== | |||
There are various combinations of outsourced maintenance and the planner needs to be very aware of the different impact these combinations might have on internal staffing levels. A phased approach according to equipment risk level should form the baseline for implementation. | |||
It would be impractical and not cost-effective to include all equipment in a preventive maintenance plan; if the risk is acceptable then the specific equipment should not form part of an IPM plan and only reactive maintenance should be performed. We can divide the equipment list into groups for specific action, as shown below (sub-types have been removed, e.g. ''Radiographic Units, Dental, Extraoral'' and ''Radiographic Units, Dental, Intraoral'' are covered by the term ''Radiographic Units, Dental''[BvR1] ) for the sake of brevity; the full lists are available on request. For each equipment type, there are established procedures and protocols for what needs to be done – these are available from organisations such as ECRI. | |||
'''A. High-risk with high maintenance intensity''' – typical outsourcing with comprehensive service contracts and short-term implementation: | |||
o Anesthesia Units | |||
o Angioplasty Systems | |||
o Apheresis Units | |||
o Autotransfusion Units | |||
o Brachytherapy Systems | |||
o Circulatory Assist Units, Cardiac, Intra-Aortic Balloon | |||
o Collimators, Radiographic | |||
o Densitometers, Bone[BvR2] | |||
o Detectors, Fluid Level, Heart-Lung Bypass Unit | |||
o Heart-Lung Bypass Units | |||
o Heat Exchangers, Heart-Lung Bypass | |||
o Hemodialysis Units | |||
o Peritoneal Dialysis Units | |||
o Positive Airway Pressure Units, Continuous | |||
o Pulsatile Pressure Generators, Heart-Lung Bypass | |||
o Radiographic Systems/Units | |||
o Radiographic/Fluoroscopic Systems/Units | |||
o Radiotherapy Beam Block Shaping Systems | |||
o Radiotherapy Simulation Systems | |||
o Radiotherapy Systems, Linear Accelerator | |||
o Scanning Systems, Computed Tomography | |||
o Scanning Systems, Gamma Camera | |||
o Scanning Systems, Magnetic Resonance Imaging | |||
o Stereotactic Systems | |||
o Ventilators. | |||
'''B. High-risk with medium maintenance intensity''' – typical outsourcing of major services, verification and inspections performed by internal staff and short-term implementation: | |||
o Defibrillators | |||
o Echocardiographs | |||
o Incubators | |||
o Inflators, Angioplasty Balloon | |||
o Lasers | |||
o Pacemakers, Cardiac, External, Invasive Electrodes | |||
o Phototherapy Units | |||
o Programmer/Testers, Implantable Cardiac Pacemaker | |||
o Scanning Systems, Ultrasonic | |||
o Phantoms, Computed Tomography | |||
o Nerve Locators | |||
o Stimulators, Electrical | |||
o Valves, Positive End-Expiratory Pressure. | |||
'''C. High, high risk with low maintenance intensity''' – typical reactive maintenance performed by internal staff with parts-only contracts as possibility and medium- to long-term implementation: | |||
o Angioscopes | |||
o Brachytherapy Applicators/Sources | |||
o Extractors, Plasma | |||
o Masks, Resuscitator | |||
o Radioisotope Transfer Units | |||
o Resuscitators, Pulmonary, Manual, Reusable | |||
o Transducers, Ultrasonic. | |||
'''D. Medium-risk with high maintenance intensity''' - typical outsourcing of major services, verification and inspections performed by internal staff and medium- to long-term implementation: | |||
o Aerators, Ethylene Oxide | |||
o Analysers, Laboratory | |||
o Cabinets, Biological Safety | |||
o Cameras, Identification | |||
o Chromatography Systems | |||
o Cleaners, Bedpan | |||
o Compressors | |||
o Computer Aided Detection Systems, Papanicolaou Smear | |||
o Detectors, Air Bubble/Foam, Heart-Lung Bypass Unit | |||
o Disinfecting Units, Liquid, Flexible Endoscope | |||
o Injectors, Contrast Media | |||
o Laminar Air Flow Units | |||
o Lithotripters | |||
o Microscopes, Light, Operating | |||
o Microwave Therapy Systems, Tissue Ablation, Prostatic | |||
o Pill Counters, Automatic | |||
o Printers, Dry-Processing | |||
o Radiofrequency Therapy Systems | |||
o Sterilising Units | |||
o Stools, Adjustable, Dentistry | |||
o Tables, Imaging, Ultrasound | |||
o Tables, Operating | |||
o Telemanipulation Systems, Surgical | |||
o Thrombometers | |||
o Tissue Processors | |||
o Urodynamic Measurement Systems. | |||
'''E.''' '''Medium-risk with medium/low maintenance intensity''' - typical maintenance performed by internal staff and long-term implementation. | |||
'''F.''' '''All low-risk equipment –''' reactive maintenance only. | |||
===Computerised maintenance management system (CMMS)=== | |||
In most modern health-care facilities, the number of pieces of medical equipment and the number of service events are so large that keeping and organising this information can only be done by a computer system. Thus, a computerised maintenance management system (CMMS) can be very useful in managing the medical equipment maintenance programme. | |||
A CMMS is made up of fields, tables and modules populated with data from the clinical engineering or medical equipment department of a given facility. Using a CMMS, critical data can be accessed, manipulated and analysed using user-friendly interfaces. Reports can be generated from the system to help policy-makers reach decisions regarding health technologies. However, it is important to take into consideration multiple factors when deciding to adopt and develop a CMMS. Factors such as financial and technical resources are important when determining whether to purchase a commercial product, use open-source software, or to develop a system locally. Implementation requires proceeding through a number of phases that will allow the system to be planned comprehensively. By completing this multistep process, the options for deployment will be thoroughly evaluated; a suitable package will be selected, installed and customised; data will be entered; and training on the CMMS will be provided. | |||
For organisations with the appropriate resources to implement this tool, a CMMS can be very beneficial. It is a highly flexible tool that when properly implemented has the ability to transform the management of medical equipment while also improving the availability and functionality of the technology required to prevent, diagnose and treat illness. | |||
A CMMS can be used to: | |||
*standardise and harmonise information within a HTM programme; | |||
*assist in the planning and monitoring of inspection and preventive maintenance, and schedule and track repairs; | |||
*monitor equipment performance indicators such as mean time between failures, downtime and maintenance costs for individual or equipment groups of the same model, type or manufacturer; | |||
*monitor clinical engineering staff performance indicators such as repeated repairs by the same staff member for the same problem, average downtime associated with individuals, and productive work time for individuals or groups; | |||
*generate reports that can be used to plan user training programmes based on equipment failure trends in certain departments or health facilities; | |||
*host libraries of regulatory requirements and safety information; | |||
*generate the appropriate documentation for accreditation by regulatory and standard organisations; and | |||
*generate reports to assist in the monitoring and improvement of the productivity, effectiveness and performance of HTM | |||
====CMMS fields and tables==== | |||
A field is a single piece of information, for example an “equipment serial number”. A table is a collection of related fields; for example, an equipment location table might be made up of the fields “building”, “department” and “room” where a piece of equipment is stored. | |||
To avoid long descriptive text, it is useful to develop a comprehensive, consistent and simple coding system for the various activities of the database. A single code is a field, and a collection of fields can be organised into tables. Coding of tables can be developed for equipment inventory, personnel, maintenance procedures and equipment locations. | |||
Commercial CMMS packages normally have a set of generic codes that can be adapted or customised according to the needs of the health facility. For “equipment type” coding, standard nomenclature such as the Universal Medical Device Nomenclature System and the Global Medical Device Nomenclature System should be considered. Implementing appropriate nomenclature can also facilitate the management of alerts and vigilance reports. A generic list of fields that are commonly included in a CMMS is provided in '''''Table 7'''''. | |||
Table 7: '''List of generic fields for a CMMS''' | |||
{| class="wikitable" | |||
|Item | |||
|Fields | |||
|- | |||
|Equipment type | |||
|Equipment type | |||
|- | |||
| | |||
|Inspection and preventive | |||
|- | |||
| | |||
| maintenance (IPM) procedures | |||
|- | |||
| | |||
|IPM frequency | |||
|- | |||
| | |||
|Risk level | |||
|- | |||
| | |||
|Responsible staff | |||
|- | |||
|Equipment model | |||
|Model number | |||
|- | |||
| | |||
|Serial number | |||
|- | |||
| | |||
|Parts list | |||
|- | |||
| | |||
|Parts code and name | |||
|- | |||
| | |||
|IPM procedures | |||
|- | |||
|Manufacturer | |||
|Manufacturer code and name | |||
|- | |||
| | |||
|Supplier code and name | |||
|- | |||
| | |||
|Manufacturer email, telephone and address | |||
|- | |||
| | |||
|Supplier email, telephone and address | |||
|- | |||
| | |||
|Manufacturer contact name | |||
|- | |||
| | |||
|Supplier contact name | |||
|- | |||
|Stores/spares | |||
|Store code and name | |||
|- | |||
| | |||
|Parts code and name | |||
|- | |||
| | |||
|Parts order number | |||
|- | |||
|Staff | |||
|Employee code | |||
|- | |||
| | |||
|Employee name | |||
|- | |||
| | |||
|Employee position | |||
|- | |||
| | |||
|Access level | |||
|- | |||
| | |||
|Training details | |||
|- | |||
|Maintenance | |||
|Inventory number | |||
|- | |||
| | |||
|Work order number | |||
|- | |||
| | |||
|Service provider | |||
|- | |||
| | |||
|Service engineer code | |||
|- | |||
| | |||
|Training details | |||
|- | |||
|Service | |||
|IPM procedures | |||
|- | |||
| | |||
|IPM interval | |||
|- | |||
| | |||
|Service provider | |||
|- | |||
| | |||
|Training details | |||
|- | |||
|Health facility | |||
|Facility code and name | |||
|- | |||
| | |||
|Area | |||
|- | |||
| | |||
|Department | |||
|} | |||
====CMMS modules==== | |||
A module is a collection of tables and data screens. The inventory module, for example, is made up of the “equipment type” table, the “manufacturer information” table and the “equipment location” table. The following lists the basic modules of a CMMS package: | |||
i. Equipment inventory module. | |||
ii. Spare parts inventory and management module. | |||
iii. Maintenance (repair) module. | |||
iv. Contract management module. | |||
v. Preventive maintenance module. | |||
vi. Reporting module. | |||
====CMMS implementation==== | |||
Clinical engineering staff must be included in the entire CMMS planning and implementation process, as shown in '''''Figure 4''''' below and described thereafter. | |||
Figure 4: '''CMMS planning & implementation process''' | |||
[[File:CMMS planning & implementation process.png|thumb|none|720x720px]] | |||
'''Phase 1 - Evaluation''' | |||
It is important to conduct a feasibility study to evaluate and assess the need for a CMMS. During this phase, a complete analysis is conducted and the scope of the system is defined. | |||
'''Phase 2 - Selection''' | |||
Once specifications for a system have been identified, an appropriate package can be selected. It may be one that is commercially available, customised to the health facility’s needs or designed specifically for the user. | |||
'''Phase 3 – Data collection''' | |||
A comprehensive survey and analysis of all available data should be conducted before implementing the CMMS. Complete and current inventory lists as well as all other tables required by the CMMS must be available to avoid costly data collection processes. Most CMMS solutions fail due to inaccurate and incomplete data collection. | |||
'''Phase 4 – Installation''' | |||
The CMMS can be implemented as a complete system, by individual modules, by equipment type or by location. This is the decision of the clinical engineering department and will depend on the resources available and hardware configuration. | |||
'''Phase 5 – Configuration and customisation''' | |||
Configuration of the system could cover areas such as simple workflow, access and security, and user preferences. Customisation refers to the technical functional requirements of the system including custom screens and tables, facility-specific workflow and additional data fields. | |||
'''Phase 6 – Data entry''' | |||
Initial data entry <s>of</s> <ins>comprises </ins>common fields such as equipment model number, inventory code, human resources, equipment locations, manufacturer information and nomenclature classifications. User security levels and associated passwords, access levels and access types are also set at this stage. | |||
'''Phase 7 – Training''' | |||
Basic and advanced functional training can now be performed. It is important that each staff member of the clinical engineering department is fully confident and familiar with all functions of the CMMS and specific level of functionality assigned to him/her. It is useful to begin staff training in the early stages of implementation to increase staff buy-in and improve confidence. | |||
'''Phase 8 – Follow up and performance monitoring''' | |||
Continuous monitoring of the system is conducted to ensure that it is directly contributing to the improvement and effective running of the HTM programme. | |||
===Human resources=== | |||
To develop an appropriate and complete staff establishment in order to achieve the objectives of the maintenance strategy is challenging and an ongoing optimisation process – even more so under a future NHI dispensation. The model presented here can be used to determine staff requirements and plan for future development; however, actual implementation will highly depend on the following factors: | |||
*Equipment included in preventative maintenance plan. | |||
*Mixture of in- and outsourced maintenance. | |||
*Availability of operational technically qualified staff. | |||
*Availability of management and support staff. | |||
*Specific health technology department structure; centralised vs decentralised structures. | |||
*Funds available to acquire resources. | |||
The provincial reviews have indicated that most provinces follow a combination approach between Centralised and Decentralised Health Technology Department structures, idealised in Figure 5 below. | |||
The Central Maintenance Unit(s) provide a single contact point to Health Facilities while decentralised HT Departments service across multiple Health Facility Locations. The Tertiary Hospital in most cases being the exception to the rule where the HT Department services the Tertiary Hospital only and form a self-supporting unit. | |||
The Central Maintenance Units are responsible for management responsibilities which includes client relationship management, regulatory compliance, uniform standards of delivery and management of human and financial resources. | |||
Figure 5: '''Idealised health technology department structure''' | |||
[[File:Idealised health technology department structure.png|thumb|none|720x720px]] | |||
====Self-supporting (stand-alone) decentralised departments - tertiary hospitals==== | |||
Properties: | |||
*Tertiary hospitals normally have intra-hospital HT departments without provincial support. | |||
*Flat management structure – not included in staff complement above. | |||
*Manage own service contracts and spare parts. | |||
*High level of specialisation. | |||
A suggested staff complement is shown in '''''Table 8''''' (this assumes a typical asset inventory and allows for two-thirds of available time spent on maintenance), and a suggested organisational structure is shown in '''''Figure 6''''' below. | |||
Table 8: '''Typical staff complements (technical) for a self-supporting HT maintenance department''' | |||
{| class="wikitable" | |||
| | |||
| colspan="4" |'''Equipment risk level''' | |||
|- | |||
|'''Resource''' | |||
|'''C-Low''' | |||
|'''B-Medium''' | |||
|'''A-High''' | |||
|'''Combined''' | |||
|- | |||
|Electronic high | |||
|0.00 | |||
|3.82 | |||
|5.68 | |||
|9.50 | |||
|- | |||
|Electronic medium | |||
|0.44 | |||
|4.28 | |||
|0.81 | |||
|5.53 | |||
|- | |||
|Electronic low | |||
|0.03 | |||
|0.02 | |||
|0.00 | |||
|0.04 | |||
|- | |||
|Mechanical high | |||
|0.00 | |||
|1.14 | |||
|0.01 | |||
|1.15 | |||
|- | |||
|Mechanical medium | |||
|1.09 | |||
|3.12 | |||
|0.01 | |||
|4.22 | |||
|- | |||
|Mechanical low | |||
|0.28 | |||
|0.06 | |||
|0.00 | |||
|0.34 | |||
|- | |||
| colspan="4" |'''Total''' | |||
|'''20.79''' | |||
|} | |||
Figure 6: '''Typical organisational structure for a self-supporting HT maintenance department''' | |||
[[File:Typical organisational structure for a self-supporting HT maintenance department.png|thumb|none|720x720px]] | |||
====Centralised maintenance units==== | |||
There are a number of variations of the Central Maintenance Units, all depending on the specific size of the area serviced: | |||
*Provincial HT maintenance units. | |||
Servicing all levels of facilities across various districts. | |||
*District HT maintenance units. | |||
Servicing all levels of facilities within a specific district. | |||
*Facility HT maintenance units. | |||
Servicing a single facility where it’s based and a couple of smaller facilities in the direct surrounding area. | |||
The typical staffing complement will depend on the type and quantity of the facilities supported by the central HT maintenance unit. Typical staffing levels per facility based on the variables in the model are suggested in '''''Tables 9-12''''' below, and the typical structure of a central HT maintenance unit is shown in '''''Figure 7.''''' | |||
Table 9: '''HT maintenance staffing requirement (regional hospital)''' | |||
{| class="wikitable" | |||
| | |||
| colspan="4" |'''Equipment risk level''' | |||
|- | |||
|'''Resource''' | |||
|'''C-Low''' | |||
|'''B-Medium''' | |||
|'''A-High''' | |||
|'''Combined''' | |||
|- | |||
|Electronic High | |||
|0.00 | |||
|0.42 | |||
|0.78 | |||
|1.20 | |||
|- | |||
|Electronic Medium | |||
|0.08 | |||
|1.01 | |||
|0.18 | |||
|1.27 | |||
|- | |||
|Electronic Low | |||
|0.00 | |||
|0.01 | |||
|0.00 | |||
|0.01 | |||
|- | |||
|Mechanical High | |||
|0.00 | |||
|0.32 | |||
|0.00 | |||
|0.32 | |||
|- | |||
|Mechanical Medium | |||
|0.34 | |||
|0.26 | |||
|0.00 | |||
|0.60 | |||
|- | |||
|Mechanical Low | |||
|0.06 | |||
|0.01 | |||
|0.00 | |||
|0.08 | |||
|- | |||
| colspan="4" |'''Total''' | |||
|'''3.47''' | |||
|} | |||
Table 10: '''HT maintenance staffing requirements (district hospital)''' | |||
{| class="wikitable" | |||
| | |||
| colspan="4" |'''Equipment Risk Level''' | |||
|- | |||
|'''Resource''' | |||
|'''C-Low''' | |||
|'''B-Medium''' | |||
|'''A-High''' | |||
|'''Combined''' | |||
|- | |||
|Electronic High | |||
|0.00 | |||
|0.32 | |||
|0.26 | |||
|0.59 | |||
|- | |||
|Electronic Medium | |||
|0.05 | |||
|0.49 | |||
|0.15 | |||
|0.70 | |||
|- | |||
|Electronic Low | |||
|0.00 | |||
|0.00 | |||
|0.00 | |||
|0.01 | |||
|- | |||
|Mechanical High | |||
|0.00 | |||
|0.15 | |||
|0.00 | |||
|0.16 | |||
|- | |||
|Mechanical Medium | |||
|0.16 | |||
|0.60 | |||
|0.00 | |||
|0.76 | |||
|- | |||
|Mechanical Low | |||
|0.03 | |||
|0.01 | |||
|0.00 | |||
|0.04 | |||
|- | |||
| colspan="4" |'''Total''' | |||
|'''2.25''' | |||
|} | |||
Table 11: '''HT maintenance staffing requirements (community health centre)''' | |||
{| class="wikitable" | |||
| | |||
| colspan="4" |'''Equipment Risk Level''' | |||
|- | |||
|'''Resource''' | |||
|'''C-Low''' | |||
|'''B-Medium''' | |||
|'''A-High''' | |||
|'''Combined''' | |||
|- | |||
|Electronic High | |||
|0.00 | |||
|0.05 | |||
|0.20 | |||
|0.25 | |||
|- | |||
|Electronic Medium | |||
|0.05 | |||
|0.13 | |||
|0.08 | |||
|0.25 | |||
|- | |||
|Electronic Low | |||
|0.00 | |||
|0.00 | |||
|0.00 | |||
|0.01 | |||
|- | |||
|Mechanical High | |||
|0.00 | |||
|0.03 | |||
|0.00 | |||
|0.03 | |||
|- | |||
|Mechanical Medium | |||
|0.16 | |||
|0.08 | |||
|0.00 | |||
|0.24 | |||
|- | |||
|Mechanical Low | |||
|0.10 | |||
|0.00 | |||
|0.00 | |||
|0.11 | |||
|- | |||
| colspan="4" |'''Total''' | |||
|'''0.88''' | |||
|} | |||
Table 12: '''HT maintenance staffing requirements (large clinic, with maternity)''' | |||
{| class="wikitable" | |||
| | |||
| colspan="4" |'''Equipment Risk Level''' | |||
|- | |||
|'''Resource''' | |||
|'''C-Low''' | |||
|'''B-Medium''' | |||
|'''A-High''' | |||
|'''Combined''' | |||
|- | |||
|Electronic High | |||
|0.00 | |||
|0.03 | |||
|0.02 | |||
|0.05 | |||
|- | |||
|Electronic Medium | |||
|0.01 | |||
|0.05 | |||
|0.01 | |||
|0.07 | |||
|- | |||
|Electronic Low | |||
|0.00 | |||
|0.00 | |||
|0.00 | |||
|0.00 | |||
|- | |||
|Mechanical High | |||
|0.00 | |||
|0.04 | |||
|0.00 | |||
|0.04 | |||
|- | |||
|Mechanical Medium | |||
|0.02 | |||
|0.03 | |||
|0.00 | |||
|0.05 | |||
|- | |||
|Mechanical Low | |||
|0.02 | |||
|0.00 | |||
|0.00 | |||
|0.02 | |||
|- | |||
| colspan="4" |'''Total''' | |||
|'''0.23''' | |||
|} | |||
Figure 7: '''Typical structure for a central HT maintenance unit''' | |||
[[File:Typical structure for a central HT maintenance unit.png|thumb|none|720x720px]] | |||
'''Properties:''' | |||
*Size depends on number of facilities. | |||
*Specialisation not as advanced as in tertiary structure. | |||
*Staff at central location and/or remote locations. | |||
*Specific functions at central to support remote staff. | |||
*Resources at remote locations can be increased with staff from central location to relieve short period demand(s). | |||
*Administration and planning of all activities from central location. | |||
==Replacement, decommissioning and disposal== | |||
===Introduction=== | |||
*Planning for replacement of a device should commence almost at the time of the original purchase to allow adequate time for budgeting and to prevent interruptions or disruption of services. Planned replacement can be based on the projected life-span of the device locally. | |||
*Decommissioning should be carried out systematically with set procedures and processes of certification, also ensuring that services are not interrupted. | |||
*Disposal of devices should be carried out systematically with set procedures and processes, with safety being a prime concern. | |||
*Additional precautions must be taken for disposal of special devices, e.g. radioactive devices. | |||
===Replacement of equipment=== | |||
Replacement is necessary because all equipment has a finite life expectancy. This lifespan will depend upon the type of equipment, and the types of technology contained within it. For example, five years might be the typical life for an ECG monitor, 10 years for a suction pump, 15 years for an operating table, and 20 years for an electricity generator. Once the equipment reaches the end of its life no amount of intervention (such as maintenance) will be effective, and the only option will be to replace it. International guidance on equipment lifetimes is available, but experience has shown that lifetimes for individual items are crucially dependent on their utilisation, care and maintenance. | |||
If replacement of equipment is not planned for, the health service delivered to the public may deteriorate. If equipment is not replaced at the end of its life, there will be: | |||
*an uneven standard of reliability among the equipment in a facility or service, and | |||
*a general deterioration in: | |||
- performance; | |||
- safety; | |||
- dependability; and | |||
- availability for use. | |||
For all medical devices and equipment, a stage is reached at which replacement must be considered. If any of the following seven criteria apply, the medical device/equipment is no longer serviceable: | |||
*Worn out beyond economic repair. | |||
*Damaged beyond economic repair. | |||
*Unreliable (check service history). | |||
*Clinically or technically obsolete. | |||
*Spare parts no longer available. | |||
*More cost-effective or clinically effective medical devices/equipment have become available. | |||
*Unable to be cleaned effectively prior to disinfection and/or sterilisation. | |||
If the medical device/equipment survives this test, a date should be set for re-testing (e.g. in a year's time). | |||
Each facility should replace equipment '''for''' valid reasons only, which should be defined. '''''Box 14''''' lists some such reasons and suggests criteria for condemning and replacing equipment. Equipment must NOT be condemned and/or disposed of on the strength of a Report or Certificate issued by the manufacturer, agent, supplier or contractor. | |||
Box 14: '''Example of valid reasons for condemning and replacing equipment''' | |||
{| class="wikitable" | |||
|'''Valid replacement criteria''' | |||
i. Equipment will be replaced '''only''' when one of the following valid reasons has been fulfilled: | |||
a. it is worn out beyond repair (has reached the end of its natural life); | |||
b. it is damaged beyond repair; | |||
c. it is unreliable – faulty, old, unsafe; | |||
d. it is clinically or technically obsolete; | |||
e. spare parts are no longer available; and | |||
f. it is no longer economical to repair. | |||
'''and''' one of the following valid reasons has also been fulfilled: | |||
g. utilisation statistics are available to show that it is still required; and | |||
h. a demonstrated clinical or operational need still exists. | |||
ii. Equipment will not be replaced simply because: | |||
*it is old; | |||
*staff do not like it; and | |||
*a newer model has arrived on the market. | |||
'''Judging when it is time to condemn equipment''' | |||
Senior maintenance staff need to study the equipment, and judge: | |||
*whether the equipment fulfils any of the valid replacement criteria (see above); | |||
*whether the equipment has outlived its (internationally/locally) advised typical “lifetime”; | |||
*the equipment’s track record and state of health, as documented in its service history records; and | |||
*whether it will be necessary to override the average expected lifespan and condemn the equipment early, or even to extend the lifespan of the equipment. | |||
For expensive equipment, it may be helpful to obtain an evaluation from the supplier. | |||
|} | |||
===Decommissioning and disposal of equipment=== | |||
Formal procedures must exist for condemning and disposal of equipment. Failure to dispose of equipment properly could result in the following: | |||
*graveyards of abandoned equipment piling up around health facilities; | |||
*departments, store rooms, cupboards, and workshops full of old equipment; and | |||
*previously condemned equipment ending up back on the wards and being re-used. | |||
Once equipment has been condemned, a formal policy is needed to oversee its disposal. This should cover: | |||
*how it should be disposed of safely; | |||
*how it can be disposed of as promptly as possible; | |||
*how it can be disposed of in an environmentally sound way according to a “waste management and hygiene plan”; and | |||
*how useful spare parts can be stripped off before the equipment is disposed of. | |||
Note: The practice of selling intact condemned medical equipment to contractors is strongly discouraged. Where possible and appropriate, the condemned medical equipment must be destroyed and sold as scrap to a metal dealer. | |||
'''''Flowchart 11''''' below describes the typical decommissioning process. | |||
The condemning and disposal of equipment should trigger the purchase of a replacement piece of equipment. It is preferable to plan for replacements before they are needed and, where possible, likely replacement needs should be identified within the annual equipment inventory update and annual plans. These activities should be timed to take place ahead of the next procurement cycle, which usually takes place annually. | |||
In summary, to replace and dispose of equipment it is necessary to have the following: | |||
technical skills to identify those items ready for replacement; | |||
*good procurement practices which enable financing and purchase of replacement items in good time; | |||
*courage and determination to take equipment out of service when necessary, even if the users want to keep using it; | |||
*a formal method for condemning equipment; | |||
*a formal method for disposing of the equipment, safely and in an environmentally sound way; and | |||
*a formal method so that the disposal of equipment triggers the purchase of a replacement item. | |||
Flowchart 11: '''Steps in a typical decommissioning process''' | |||
[[File:Flowchart 11 - Steps in a typical decommissioning process.PNG|thumb|none|720x720px]] | |||
== Monitoring & evaluation of the HTM system== | |||
This document has outlined the building blocks of an effective, evidence-based and responsive Health Technology Management (HTM) system. This system – in its pivotal contributing role to safe, cost-effective, accessible and quality healthcare service delivery - needs to be monitored and evaluated on an ongoing basis at all levels of care delivery. | |||
'''Annex III''' suggests a set of '''Standards''' that align with the sections in this document; they also underline the need for a clear statement of vision and intended performance. They also indirectly point to the need for adequate and proper resourcing – at all levels of delivery – of the structures, processes, activities and human capital that together constitute an HTM system. | |||
'''Annex III''' also contains a list of HT-related '''Indicators''' that could be used to measure/assess compliance with these Standards as well as indicate levels of performance in their own right. | |||
These HT-related Standards and Indicators are particularly relevant with respect to the newly established Office of Standards Compliance of the Department of Health and the associated National Core Standards for Health Facilities. They will also be very useful as bridging links between the public and the private sectors as we move towards an NHI dispensation. | |||
=Annexures= | |||
==Annex I: Sample Generic Equipment Specification: Infant Incubator== | |||
''NB: The specification is intended to indicate the range of issues addressed as well as the level of detail, clarity of language and formatting/layout typically associated with a good technical specification; however, since this is being used for illustrative purposes, many clauses have been omitted for the sake of brevity.'' | |||
1. APPLICABLE DOCUMENTS | |||
The specification should be read in conjunction with the “Technical and Environmental Data Sheet”, and all goods offered must conform to the details specified in it and be able to function in the prevailing conditions described. | |||
2. REQUIREMENTS | |||
2.1 GENERAL DESCRIPTION | |||
To supply: ONE unit to provide a suitable environment conducive for nursing ill, premature, and underweight babies. | |||
2.2 OPERATIONAL REQUIREMENTS | |||
''Note: supplier to complete “Reply” and “Remarks” sections.'' | |||
2.2.1 There shall be a trolley base with four swivel wheels, at least two lockable. | |||
2.2.2 The incubator shall fit securely onto the trolley. | |||
2.2.3 The incubator base shall house the power compartment, fan and humidifier tank. | |||
2.2.4 The infant compartment shall have a base, mounted above the humidifier tank and fan, which is large enough to allow the unimpeded handling of the infant. Base shall have smooth, easy to clean surfaces. | |||
2.2.5 The baby tray shall be mounted on the infant compartment base and shall be tiltable. | |||
… | |||
2.2.8 The infant compartment shall have a transparent canopy that forms four sides and the roof. | |||
2.2.9 The canopy shall be hinged along one side so that it can be swung up to provide free access to the bed. | |||
… | |||
2.2.15 The air shall be drawn into the incubator through an easily removable bacteria filter capable of removing, with an efficiency of 99%, particles of the size down to 0.5 micron diameter. | |||
2.2.16 The air shall be circulated by means of a fan. | |||
2.2.17 The circulated air shall maintain slight positive pressure in the infant compartment such that enough stale air escapes from the hood to prevent an undesirable and dangerous carbon dioxide accumulation inside blood. | |||
2.2.18 The hood shall have inlet holes for access by oxygen and feeding tubes. | |||
… | |||
2.2.21 There will be an air temperature sensor mounted on the inside of the canopy. | |||
2.2.22 The incubator shall be equipped with heating elements of the totally enclosed metal-clad type and a thermostat capable of controlling the temperature in the infant compartment over a specific temperature range. | |||
2.2.23 The incubator shall be equipped with a reliable pre-set high-temperature cut-out that operates completely independently from the thermostat and that disconnects the heating circuit from the electricity supply if, as a result of heating from any source (including direct sunlight or nearby heaters), the temperature in the infant compartment exceeds 39˚C. Any relay forming part of this circuit shall be arranged to be fail-safe. | |||
2.2.24 Temperature range of 34-39 degrees Celsius, in increments of 0.1 degree. | |||
2.2.26 The airflow alarm shall be activated if the airflow is obstructed (due to fan failure or total air circulation failure). The activation of the alarm shall cause a cut off of the heating elements. It shall be mains operated audible and visual. | |||
2.2.27 The high temperature alarm shall be activated if air temperature in the canopy exceeds 39˚C. It shall be mains operated audible and visual. | |||
2.2.28 The power failure alarm shall give warning of any interruption of the electric power supply to the incubator. The alarm shall be operated from a battery of the nickel cadmium type that is housed in the power compartment and is continuously trickle-charged when the power is switched on. The alarm shall be audible and visual. | |||
2.2.29 Single-phase power supply of 220-240 Vac, 50Hz. | |||
2.2.30 To be able to withstand mains supply voltage fluctuations of +/- 10%, and mains supply frequency fluctuations of +/- 10%. | |||
2.2.31 The incubator shall be equipped with a 3-metre non-kinking type flexible mains lead, fitted with a three pin (square)13A plug. The mains connector to be detachable locking type. | |||
2.2.32 The humidifier tank will consist of a water reservoir, water inlet port, and water outlet drain constructed in such a way that once drained a residue puddle of water cannot remain in the reservoir. | |||
2.3 PHYSICAL CHARACTERISTICS | |||
2.3.1 The trolley to be of metallic tubular frame of such dimensions and wall thickness as to give acceptable strength and rigidity. It shall have a polyester powder coating finish. | |||
2.3.2 The casters will be of a minimum size of 100mm. | |||
2.3.3 The incubator base shall be of metal construction with a polyester powder coating finish. | |||
2.3.4 The power compartment shall be of metal and so designed that the mechanical and electrical equipment within it is adequately protected against mechanical damage and the ingress <s>i</s><ins>o</ins>f water and cleaning fluids. | |||
… | |||
2.3.7 The bed tray and support shall be of corrosion-resistant material. | |||
… | |||
2.3.10 It should be possible to fully dismantle the equipment for cleaning purposes; and all parts will be easily cleaned. | |||
2.4 SAFETY FEATURES | |||
2.4.1 The unit must be manufactured to conform to the IEC safety standard 60101 for medical electrical equipment 2.4.2 Safety Classification: Type B. | |||
3. ACCESSORIES AND CONSUMABLES | |||
3.1 The trolley base to contain a storage compartment with latching doors. | |||
3.2 An IV pole shall be attached to the trolley. | |||
3.3 An oxygen cylinder holder shall be attached to the trolley. | |||
… | |||
3.6 Supply all necessaries for the unit to function as described. | |||
3.7 A list of each accessory and its cost must be stated. | |||
3.8 State all consumables necessary for the unit to function for two years. | |||
3.9 A list of each consumable and its cost must be stated. | |||
4. DOCUMENTATION | |||
4.1 Supply an operating manual in English for the machine. | |||
4.2 Supply a service manual in English for the machine. | |||
4.3 Supply a list of recommended spare parts required for the maintenance of the machine, | |||
5. SPARE PARTS | |||
5.1 Supply a set of only the recommended essential spare parts for 24 months for maintenance and repair. | |||
5.2 A list of each part and its price must be attached to this bid. | |||
6. DELIVERY | |||
6.3 The cost of freighting the goods by sea and road (alt: air and road) to health facility X must be stated. | |||
7. INSTALLATION/COMMISSIONING/TRAINING | |||
7.1 Full assembly and commissioning instructions must be provided for assembly and commissioning by the client, in a written format and as a video if available. | |||
7.2 The cost of commissioning by the supplier or representative must be stated. | |||
7.3 State the cost of the supplier or representative undertaking training and providing written guidelines: | |||
- in operation – for users; | |||
- in care and cleaning – for users; | |||
- in PPM – for maintenance technicians; and | |||
- in repair – for maintenance technicians | |||
8. WARRANTY | |||
8.1 A guarantee period must be stated (a minimum of 12 months from the date of commissioning). | |||
9. AFTER-SALES SUPPORT | |||
9.1 After-sales support must be available, with maintenance capabilities and facilities, and spare parts stock holdings. | |||
9.2 Details of the availability and location of spare parts must be stated. | |||
9.3 Details of the availability and location of maintenance facilities must be stated. | |||
9.4 The cost of the annual maintenance contract must be stated, detailing the range/scope of such maintenance work. | |||
10. SUMMARY OF PRICES (detailed as follows: total prices, showing options and alternatives): | |||
1. Basic unit. | |||
2. Accessories as detailed. | |||
3. Optional accessories. | |||
4. Consumables. | |||
5. Documentation. | |||
6. Spare parts for maintenance and repair for 24 months. | |||
7. Crating, delivery, insurance. | |||
8. Commissioning, training. | |||
9. Annual maintenance contract. | |||
==Annex II: Healthcare technology standards and indicators== | |||
===Standards=== | |||
The following standards are suggested in alignment with some of the key building blocks of an HTM System. | |||
====HT Planning (S1)==== | |||
There is a system in place which ensures that health technologies are properly planned and provided for in response to disease burden profiles and healthcare needs of populations served, in alignment with service delivery needs and in compliance with organisational and financial constraints. | |||
====HT Inventory (S2)==== | |||
There is a system in place which ensures that appropriate and adequate information on health technology/ medical device assets is maintained, and that this information is validated, regularly updated and accessible to HTM-related decision-making structures and processes at all levels of healthcare delivery. | |||
====HT Budgeting and financing (S3)==== | |||
There is a system in place which ensures that health technologies and related expenditures are properly budgeted for and appropriately and sustainably financed, in accordance with prescribed regulations and organisational policies. | |||
====HT Requisition (S4)==== | |||
There is a system in place which ensures that health technology requests at all levels are properly initiated, screened and collated, according to established criteria and in compliance with specified formats and processes. | |||
====HT Procurement (S5)==== | |||
There is a system in place which ensures that health technology requests, if properly drafted and considered, are translated into a transparent, criteria-driven process that ensures the acquisition of the best technology solution with due consideration of organisational needs and preferences and related criteria. | |||
====HT Receipt, testing, installation & commissioning (S6)==== | |||
There is a system in place which ensures that medical equipment, as part of the procurement process, is properly received, tested, installed and commissioned prior to use in the healthcare environment, in accordance with laid-down procedures and accepted good practice. Pre-installation site preparation or modification may be required. | |||
====HT-related training and skills development (S7)==== | |||
There is a system in place which ensures that both medical equipment users and maintenance personnel receive appropriate and ongoing training and skill development, so that healthcare interventions and procedures requiring medical equipment, devices and/or instruments are delivered safely and effectively. | |||
====HT operation (S8)==== | |||
There is a system in place which ensures that health technologies are utilised and managed optimally so as to achieve maximal benefit in terms of health outcomes, as part of quality healthcare delivery. | |||
====HT maintenance, risk management and safety (S9)==== | |||
There is a maintenance and risk management system in place that, in keeping with international and national standards and practice, ensures safety of staff and patients. | |||
===Indicators=== | |||
====Core indicators (ex WHO)==== | |||
'''I. Inputs and Processes''' | |||
'''1. Procurement budget''' | |||
'''Description: ''' | |||
Total budget spent on new acquisitions in relation to total current asset replacement value (%). | |||
'''Rationale: ''' | |||
A sufficient budget allocation is necessary in order to replace outdated assets and keep pace with rapid developments in new medical procedures and changing trends in technology. The total budget combines new acquisitions (additions to the existing asset base) and funds for the replacement of outdated and obsolete assets. The total budget combines funding from all sources. Benchmark/Target: 10% or higher. | |||
'''Measurement: ''' | |||
Evaluation of budgets, at national, sub-national or facility level, extracting the budget spent on new acquisitions, in relation to the total asset replacement value. | |||
'''Effort level: ''' | |||
Low to medium. | |||
'''Numerator: ''' | |||
Total budget spent on new acquisitions per annum. | |||
'''Denominator:''' | |||
Total current asset replacement value. | |||
'''Data collection methods/sources:''' | |||
Statistics to be provided by the health institutions administration, technical department and/or MoH. | |||
'''Periodicity: ''' | |||
Annual. | |||
'''2. Maintenance budget''' | |||
'''Description: ''' | |||
Total combined budget spent on maintenance and training in relation to total current asset replacement value (%). | |||
'''Rationale: ''' | |||
Proper maintenance of assets and training of support staff in the use and maintenance of assets is a prerequisite for effective asset management. The budget spent on maintenance and training has to be in a sound relation to the total asset replacement value. Benchmark/Target: 6% or higher. | |||
'''Measurement: ''' | |||
Evaluation of budgets, at national, sub-national or facility level, extracting the budget spent on maintenance and training, in relation to the total asset replacement value. | |||
'''Effort level: ''' | |||
Low to medium. | |||
'''Numerator: ''' | |||
Total budget spent on maintenance and training. | |||
'''Denominator:''' | |||
Total current asset replacement value. | |||
'''Data collection methods/sources:''' | |||
Statistics to be provided by the health institutions administration, technical department and/or MoH. | |||
'''Periodicity: ''' | |||
Annual. | |||
'''3. Workforce''' | |||
'''Description: ''' | |||
Number of trained technical support staff related to number of devices. | |||
'''Rationale: ''' | |||
Only with a sufficient number of qualified managerial, engineering and technical staff can adequate, high-quality health infrastructure and technology management, and thus high quality healthcare, be sustained. Benchmark: ratio in countries with high-quality CE services. | |||
'''Measurement: ''' | |||
The number of trained technical support staff per 1 000 medical devices. | |||
'''Effort level: ''' | |||
Low. | |||
'''Numerator: ''' | |||
The number of trained technical support (CE) staff. | |||
'''Denominator:''' \ | |||
Total number of medical devices supported. | |||
'''Data collection methods/sources:''' | |||
Statistics to be provided by the health institutions administration, technical department and/or MoH. | |||
'''Periodicity: ''' | |||
Annual. | |||
'''II. Outputs''' | |||
'''Asset quality''' | |||
'''Description: ''' | |||
Proportion of assets ready for service delivery in a reliable and safe manner (%). | |||
'''Rationale:''' | |||
At any given time, all assets should be in working and safe condition and be used to their full capacity. There should be no defective, unsafe or outdated assets. Benchmark: 100%. | |||
'''Measurement: ''' | |||
Inspection (audit) of all assets in regular intervals and maintaining a checklist indicating working condition, safety and usage. | |||
'''Effort leve'''l: ''' ''' | |||
Low to medium. | |||
'''Numerator: ''' | |||
Number of assets ready for service delivery - i.e. reliable and safe. | |||
'''Denominator:''' | |||
Total number of assets. | |||
'''Data collection methods/sources:''' | |||
Statistics to be provided by the health institutions administration, technical department and/or MoH. | |||
'''Periodicity: ''' | |||
Annual. | |||
'''5. Asset downtime''' | |||
'''Description: ''' | |||
Average downtime of assets following breakdown or failure (days). | |||
'''Rationale:''' | |||
The period of time that it takes the service department to restore defective assets in health facilities to their original working state should be kept to a reasonable and feasible minimum. Benchmark/Target: Minimum period of time (days). | |||
'''Measurement''': ''' ''' | |||
Statistics on downtime of all equipment (by major category/type). | |||
'''Effort level: ''' | |||
Low. | |||
'''Numerator''': | |||
Average downtime of assets following breakdown or failure (in days). | |||
'''Data collection methods/sources:''' | |||
Statistics to be provided by the health institutions administration, technical department and/or MoH. | |||
'''Periodicity: ''' | |||
Annual. | |||
'''III. Outcomes''' | |||
'''6. Service access''' | |||
'''Description: ''' | |||
Percentage of HIT-related patient referrals. | |||
'''Rationale: ''' | |||
Unnecessary referrals to other, normally higher-level facilities, as a result of asset-related problems, delay the treatment of patients and cause additional costs, thus negatively reflecting on the quality of healthcare. Benchmark/Target: No referrals (0%). | |||
'''Measurement: ''' | |||
Statistics on the total number of patients and the number of health infrastructure- and technology-related referrals. | |||
'''Effort level: ''' | |||
Low. | |||
'''Numerator: ''' | |||
Number of patients being referred to another facility due to asset problems (lack of equipment or consumables, equipment out of order, etc.). | |||
'''Denominator:''' | |||
Total number of patients making use of facility (as either out- or inpatients). | |||
'''Data collection methods/sources:''' | |||
Statistics to be provided by the health institutions, healthcare providers and MoH. | |||
'''Periodicity: ''' | |||
Annual. | |||
'''7. Service delay''' | |||
'''Description: ''' | |||
Percentage of patients unduly delayed due to asset failure. | |||
'''Rationale: ''' | |||
With adequate implementation of proper management processes, patient wait times due to asset failure will be kept to a minimum. Benchmark/Target: No delays (0%). | |||
'''Measurement: ''' | |||
Statistics on asset failure and the number of patients delayed by these failures. | |||
'''Effort level: ''' | |||
Medium. | |||
'''Numerator: ''' | |||
Number of patients with delayed diagnosis or treatment due to asset failure. | |||
'''Denominator:''' | |||
Total number of patients making use of facility (as either out- or inpatients). | |||
'''Data collection methods/sources:''' | |||
Statistics to be provided by the health centres and MoH. | |||
'''Periodicity: ''' | |||
Annual. | |||
'''IV. Impact''' | |||
'''8. Patient safety''' | |||
'''Description: ''' | |||
Rate of adverse events due to asset failure. | |||
'''Rationale: ''' | |||
HIT-related incidents and accidents sharply decrease with improving the quality of the technical support services. Asset-related adverse events can affect both staff and patients and must be notified. Benchmark/Target: No adverse events during the period of assessment. | |||
'''Measurement: ''' | |||
Evaluation of accident and incident reports. | |||
'''Effort level: ''' | |||
Medium, decreasing to low with the introduction of an accident and incident notification system. | |||
'''Numerator:''' | |||
Number of HIT-related accidents and incidents for the institution or level assessed. | |||
'''Denominator:''' | |||
Total number of patients making use of facility (as either out- or inpatients). | |||
'''Data collection methods/sources:''' | |||
Statistics to be provided by the health centres and MoH. Statistics from the accident and incident reporting system. Statistics from the WHO safe surgery checklist. | |||
'''Periodicity: ''' | |||
Annual. | |||
'''9. Staff satisfaction''' | |||
'''Description: ''' | |||
Degree of satisfaction of health workers with asset performance. | |||
'''Rationale''': ''' ''' | |||
Staff satisfaction is to a large extent related to the positive or negative contribution of asset performance to the effectiveness and efficiency of healthcare delivery. Benchmark/Target: High degree of satisfaction. | |||
'''Measurement: ''' | |||
Evaluation of questionnaires. A representative sample of health workers expresses their level of satisfaction on a scale of 1-5: (1) Very poor, (2) Poor, (3) Reasonable, (4 ) Good, (5) Excellent. | |||
'''Effort level:''' | |||
Medium. | |||
'''Numerator:''' | |||
Satisfaction score (average and range) as per agreed grading system. | |||
'''Data collection methods/sources:''' | |||
Questionnaires to be answered by a representative sample of health workers in the health institutions. | |||
'''Periodicity: ''' | |||
''' ''' Annual | |||
'''10. Financial risk''' | |||
'''Description: ''' | |||
Proportion of assets in good working condition, but not used. | |||
'''Rationale: ''' | |||
With appropriate management, all assets should be used up to their full capacity. In cases were assets are in good working condition, but not fully utilised, this is seen as a financial waste. Benchmark/Target: 0%. | |||
'''Measurement: ''' | |||
Inspection of all assets at regular intervals and maintaining a checklist indicating their use. | |||
'''Effort level: ''' | |||
Low. | |||
'''Numerator: ''' | |||
Number of assets not used (or used to less than 50% of expected capability), though in good working condition. | |||
'''Denominator:''' | |||
Total number of assets. | |||
'''Data collection methods/sources:''' | |||
Statistics to be provided by the health institution administration, technical department and/or MoH. | |||
'''Periodicity: ''' | |||
Annual.<br /> | |||
==References and selected bibliography== | |||
1. Banta D, Luce B (1993). Health Care Technology and its Assessment: An International Perspective. Oxford University Press. | |||
2. Department of Health, Republic of South Africa (2000a). A Framework for Health Technology Policies. | |||
3. Department of Health, Republic of South Africa (2004). Health Technology Assessment: Discussion Document on a Strategy for the Future. Report of Interim Steering Committee on Health Technology Assessment. | |||
4. Department of Health, Republic of South Africa (2005). National Health Technology Management Policy (draft). | |||
5. Federal Ministry of Health, Republic of Sudan (2011). Health Technology Management Policy (Medical Devices) | |||
6. Locke GP (2000). Safety and Risk Management. Course Notes, Healthcare Technology Management (HTM) Programme, University of Cape Town. | |||
7. Ministry of Health, Republic of Uganda (2009). National Medical Equipment Policy (4th edition). | |||
8. Nunziata E (2003). Workshops overview. Basic Principles and Implementation Strategy. | |||
9. Nunziata E, Poluta M (2006). Health Technology Unit: Framework for Establishment of Regional Workshops. Consultancy Report. | |||
10. Nunziata E, Poluta M (2006). Medical Device Acquisition and Procurement Policy: Draft and Background Document. Consultancy Report. | |||
11. Nunziata E, Poluta M (2012). Procedures, Standards and Norms for Healthcare Technology Lifecycle Management. Consultancy Report. | |||
12. Panerai R, Mohr B (1989). HTA Methodologies for Developing Countries. PAHO. | |||
13. Poluta M (2013). Course Notes (various), Healthcare Technology Management (HTM) Programme, University of Cape Town. | |||
14. Ridgway M, Atles LR, Subhan A ,Reducing Equipment Downtime - A New Line of Attack. (2009). J of Clin Eng, Oct/Dec issue, pp 200-4. | |||
15. Rosen R, Gabbay J (1999). Linking health technology assessment to practice. BMJ vol. 319. | |||
16. Stevens A, Milne R, Lilford R, Gabbay J (1999). Keeping pace with new technologies: systems needed to identify and evaluate them. BMJ vol. 319, Nov. | |||
17. Temple-Bird C (2000). Practical Steps for Developing Health Care Technology Policy. Institute of Development Studies, Brighton. | |||
18. Wang B, Fedele J, Pridgen B et al (2010). Evidence-Based Maintenance - Part I: Measuring Maintenance Effectiveness with Failure Codes. J of Clin Eng, Jul/Sep issue pp 132-144. | |||
19. WHO (1989). Manpower Development for a Health Care Technical Service – Report of WHO Interregional Meeting, Campinas, Brazil. | |||
20. WHO (1997). Guidelines for Healthcare Equipment Donations. | |||
21. WHO (1998). Final Report of the WHO Consultation on Physical Infrastructure and Technology. | |||
22. WHO (2001). Report of Technical Consultation on Effective Coverage in Health Systems WHO/EIP/OSD/10.01. | |||
23. WHO (2002). Safe Medical Devices. Aide-Memoire for National Medical Device Administrations. | |||
24. WHO (2008a). Strengthening Healthcare Infrastructure and Technology Management for Optimized Health Service Delivery. Luxembourg – WHO Project Technical Document. | |||
25. WHO (2008b). Measuring Health Systems Strengthening and Trends: A Toolkit for Countries. | |||
26. WHO (2010a). A Framework for National Health Policies, Strategies and Plans. Consultation Document (draft 3). | |||
27. WHO Collaborating Centre for Essential Technologies in Health (1998). The Essential Technology Package – a decision-support tool for selection and codification of equipment based on identified health interventions and delineated procedures. | |||
28. WHO. Towards a WHO Model List of Essential Medical Devices. Dept. of Essential Health Technologies. | |||
29. WHO/World Bank/GAVI/Global Fund (2009). Monitoring and Evaluation of Health Systems Strengthening – An Operational Framework. | |||
30. WHO-AFRO (1998). Guidelines for the Formulation of a National Equipment Policy. | |||
31. WHO-AFRO (1999). Health Technology Policy in the African Region – Horizon 2010. | |||
32. WHO-EMRO (1997). Resolution EM/RC44/R3 on Appropriate Health Technology. | |||
33. WHO-EMRO (2001a). Eastern Mediterranean Regional Strategy for Appropriate Health Care Technology. No. 2 in Series on Health Care Technology Management. | |||
34. WHO-EMRO (2001b). Health Care Technology Policy Formulation and Implementation. No. 3 in Series on Health Care Technology Management. | |||
35. WHO-EMRO (2001c). Health Care Technology Policy Framework. No. 1 in Series on Health Care Technology Management. | |||
36. Ziken International (2005). “How to Manage” Series for Healthcare Technology. DFID KaR Programme | |||
37. WHO Medical Device Technical Series (2011) includes: | |||
1. WHO EHT Computerised Maintenance Management System Guide | |||
2. WHO EHT Donations Guide | |||
3. WHO EHT HTA Guide | |||
4. WHO EHT Inventory Management Guide | |||
5. WHO EHT Maintenance Guide | |||
6. WHO EHT Needs Assessment for Medical Devices | |||
7. WHO EHT Procurement Process Guide | |||
Available at <nowiki>http://www.who.int/medical_devices/management_use/en/</nowiki> | |||
{{Expand}} | |||
[[Category:Crosscutting Issues]] | |||
Latest revision as of 12:22, 9 October 2020
Background
Health technology context
Health Technology covers a wide range of apparatus, consumables, devices, equipment and instruments that would require many volumes of documents to cover effectively. This document, as part of the broader IUSS Norms and Standards project, aims to look at the key elements of Health Technology and its management as it manifests in healthcare facilities. The document specifically aims at framing the subject of Health Technology within the infrastructure development and operations domain.
The various definitions of Health Technology are listed in the Glossary but it is important to note that this document focuses on durable medical equipment and related consumables used in healthcare facilities. Hospital plant and machinery, normally associated with the building and most often installed as part of the building, are dealt with in various other documents in the IUSS document bouquet, either under the respective hospital engineering disciplines or under the respective clinical service area.
Application of the Guidelines
This document, published under the Health Facility guides, would be useful for practitioners of Health Technology Management both at the level of an individual facility and for groups of facilities (for example, in a district) and at any level of care. It would also be of great value for HTM practitioners and facility managers in general who might lack the first-hand experience of this relatively specialised field. In many cases the guidelines show the way towards developing specific and applicable solutions rather than being prescriptive. This approach is necessary to ensure that they remain sufficiently generic to be applied across a broad spectrum of scenarios. The document would also be of interest to health infrastructure professionals who might need specific insight into the domain of Health Technology and its management, recognising that technology and infrastructure are closely inter-related and will become ever more so in future healthcare systems.
This document should be considered in the context of the solid platform provided by the Health Technology Policy and the Health Facilities Planning Directorates of the National Department of Health. This includes the Framework for Health Technology Policies which outlines the vision of a National Health Technology System; the draft Health Technology Management Policy document which inter alia outlines the organisational and structural requirements at all levels of governance pertaining to such a system; the Health Technology Strategy which outlines specific objectives and activities, with associated timeframes, for establishing a National Health Technology System; the Report of the Interim Steering Committee on Health Technology Assessment, which underlines the importance of having formal processes in place to assess issues of health technology cost-effectiveness, access, service-fit and social impact, amongst other “evaluative dimensions”; and lastly the draft Medical Device Regulations and associated background documents which – in their final form – will be promulgated under the South African Health Products Regulatory Agency (SAHPRA) Act .
Life cycle of Health Technology
Planning
Introduction
Planning and budgeting are often considered jointly since planning – for it to be effective – needs to take place within the context of policy, financial, and other constraints. Box 1 shows the overall process.
BOX 1: The planning and budgeting process (start-up)
| Steps | People responsible | Actions |
|---|---|---|
| Plan and budget within the framework of guidance and direction from the national level. | Health service managers at national level in consultation with managers at other levels. | Framework requirements
|
| Increase the availability of planning skills for equipment at all service levels, by developing planning “tools” through one-off exercises. | HTM working groups and sub-groups.
Finance officers. |
Knowing where one is starting from
|
| Health management teams.
|
Knowing where one is headed
| |
| Ensure realistic estimates are made for all equipment-related allocations at all service.levels, by using budgeting “tools” to calculate expenditures required. | HTM working groups and sub-groups.
|
Capital budget calculations
|
| Ensure realistic estimates are made for all equipment-related allocations at all service levels, by using budgeting “tools” to calculate expenditures required. |
HTM managers and their teams. Heads of section.
|
Recurrent budget calculations
|
| Use the tools to make long-term plans and budgets |
|
Long-term planning
|
| Review the plans and budgets annually, and monitor progress in order to improve planning and budgeting. |
HTM working groups and sub-groups. |
Annual planning
|
| Review the plans and budgets annually, and monitor progress in order to improve planning and budgeting. |
Heads of department and HTM managers.
teams. |
Monitoring progress
|
For healthcare technology to be managed effectively, a clear idea of healthcare delivery goals and targets is needed, as well as the context in which the technology is operating. Equipment should not be viewed in isolation – it is there for a purpose, and must be managed according to set objectives. For effective planning, access to a wide range of information and reference materials is needed, as well as a clear vision of the direction in which the health service is headed, plus identification of what equipment is required to help achieve the health service goals.
To inform the technology part of the debate, the HTM working group (at each level) should consider the equipment implications of the healthcare interventions suggested, and then offer technical advice to their health management team. Box 2 and Checklist 1 show some of the issues that the national, province/district- and facility level HTM working groups, respectively, should consider.
BOX 2: Baseline information on medical devices
| Medical device situation | Considerations | Result |
|
|
|
CHECKLIST 1: Equipment considerations for vision at national level
| Issues | Example |
|---|---|
| What expansion of services is necessary or feasible? |
|
| What are the implications in terms of staff, skills, resources, patient referral networks? |
|
| Are desired expansions financially affordable? |
|
| Do the services suggested fit into the overall health service in the country? |
|
Planning and budgeting when starting out
Box 3 shows the minimum requirements for a scenario for an HTM system in its infancy, where the initial focus is not on long-term forward planning, but concentration on planning and budgeting on a yearly basis. As the system matures, other elements for forward planning can be added.
Box 3: Minimum planning and budgeting requirements
| Planning and budgeting element | If just starting out |
| 1. Equipment inventory
2. Stock value estimates 3. Budget lines for equipment expenditures. 4. Usage rates for equipment-related consumable items. 5. Reference materials. 6. Developing the vision of service delivery for each facility type. 7. Standard Equipment Lists. 8. Purchasing, donations, replacement, and disposal policies. 9. Generic equipment specifications and technical data. 10. Capital budget calculations. 11. Recurrent budget calculations. 12. Equipment development plan. 13. Equipment training plan. 14. Core equipment expenditure plan. 15. Core equipment financing plan. 16. Annual equipment planning and budgeting. 17. Monitoring progress. |
1. Essential to have.
2. Useful to carry out this exercise later on when rough estimates needed for long-term forward planning. 3. This alteration to budget layout can be done later, but it will help with analysis. 4. Useful to do this exercise as it helps with calculation of specific (annual) estimates. 5. These can be developed over time. 6. Should have an understanding of this, even if full exercise not undertaken. 7. Initially, list of urgent equipment needs drawn up by departments can be used. Later on, Standard Equipment Lists obtained elsewhere can be used. 8. Essential to have. 9. Initially learn from others. Later, develop own. 10. Initially learn how to make specific (annual) estimates; only learn the rough estimation methods when undertaking long-term planning. 11. Initially learn how to make specific (annual) estimates. Only learn the rough estimation methods when undertaking long-term planning. 12. Use the basic equipment development planning process only, and only apply it to the short term. 13. Develop a straightforward one for the short term 14. Initially only plan annually (see below). 15. Initially only plan annually (see below). 16. Create annual actions plans and an equipment budget showing income and expenditure. 17. Undertake the basic elements only – progress with annual plans and tools, coping with emergencies, providing feedback. |
Needs assessment
Standard lists of equipment
Once the vision for the direction of health service delivery has been developed, the healthcare interventions and procedures to be offered will be known. Based on this information, the Essential Service Packages can be developed; these will translate the vision into:
- human resource requirements, and training needs;
- space requirements, and facility and service installation needs; and
- equipment requirements.
The tool used in the process of defining what equipment is needed for each healthcare intervention is the Standard Equipment List. This is:
- a list of equipment typically required for each healthcare intervention (such as a healthcare function, activity, or procedure). For example, health service providers might list all equipment required for eye-testing, delivering twins, undertaking fluoroscopic examinations, or for testing blood for malaria;
- organised by activity space or room (such as reception area or treatment room), and by department;
- developed for every different level of healthcare delivery (such as district, province) since the equipment needs will differ depending on the vision for each level;
- usually made up of everything including furniture, fittings and fixtures, in order to be useful for planners, architects, engineers and purchasers, and
- a tool which allows healthcare managers to establish if the Vision is economically viable.
The Standard Equipment List must reflect the level of technology of the equipment. It should describe only technology that the facility can sustain (in other words, equipment which can be operated and maintained by existing staff, and for which there are adequate resources for its use). For example a department could have:
- an electric suction pump or a foot-operated one;
- a hydraulic operating table or an electrically controlled one;
- a computerised laundry system or electro-mechanical machines; and
- disposable syringes or re-usable/sterilisable ones.
It is important that any equipment suggested:
- can fit into the rooms and space available. Reference should therefore be made to any building norms defining room sizes, flow patterns, and requirements for water, electricity, light levels and so on;
- has the necessary utilities and associated plant (such as the power, water, waste management systems) available for it on each site - if such utilities are not available, it is pointless planning to invest in equipment which requires these utilities in order to work; and
- can be operated and maintained by existing staff and skill levels, or for which the necessary training is available and affordable.
Usefulness of the standard equipment lists
A Standard Equipment List is an aid to the planning process. In order to plan what equipment to purchase, awareness of any shortfalls in equipment is needed. To determine such shortfalls, the Equipment Inventory needs to be compared with the Standard Equipment List. This will indicate whether any equipment is currently missing or needs to be purchased. Thus, the Standard Equipment List will assist in determining what equipment is:
- necessary;
- surplus;
- extravagant; and
- missing
in relation to the Vision for the service.
Responsibility for developing the Standard Equipment Lists varies from country to country. It is most important that this task is undertaken by a multidisciplinary team, so that decisions benefit from the skills and views of many disciplines, not just one or two. The health service planners at central/national level should consider developing Standard Equipment Lists in collaboration with staff from each level of the service, as indicated in Box 4 below.
| Who/which level? | Takes what action? |
| HTM working group at each level | Organises special meetings of different types of staff to work on the Standard Equipment List. Then reports back to the Health Management Teams. |
| National level | Takes the first step and runs specific exercises to establish the Standard Lists of Equipment for each clinical and support area, at each operational level. |
| Province/district level | Takes the second step and adjusts the list on a regional/district basis to cover local variations. |
| Facility level | Takes the third step and assesses:
- how they can provide the healthcare interventions; and - what numbers of equipment they require depending on how they organise their work. Organisational decisions influence the quantity of equipment. For example, the timing of clinics can reduce or increase the workload in the laboratory. Before ordering new equipment, its level of use will need to be assessed. |
Equipment development plan
Especially for an HTM system that is not mature, it is essential to have important to have [BvR1] an equipment development plan; one suggestion for the development planning process is presented in Flowchart 1 below.
Flowchart 1: The basic equipment development planning process
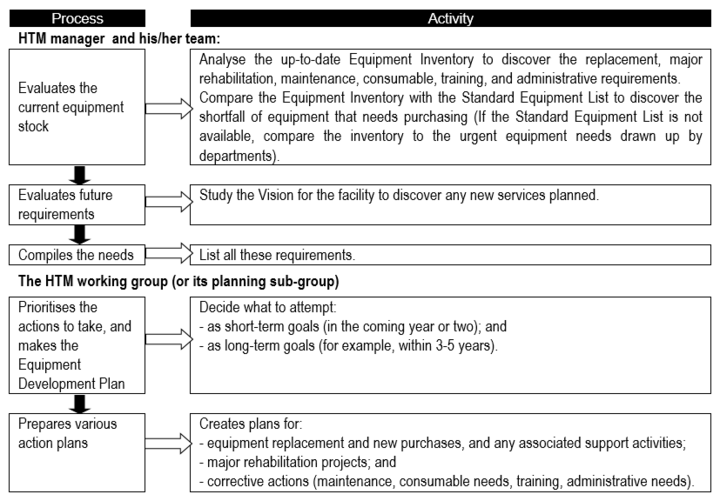
Prioritising and appraisal of options
It is equally important, in the context of needs assessment, to critically examine the process and its outputs and outcomes. Checklist 2 below poses some key questions for consideration by the appropriate stakeholder/s.
Checklist 2: Key questions for prioritising and appraisal of options
Impact
|
Changeability
|
Acceptability
|
Resource feasibility
|
Consumables
Consumables are a major contributor to equipment-related cost of ownership, and form a significant portion of recurrent and operational expenditure. It is therefore important to establish the consumables needs so that adequate provision is made and service delivery is not interrupted. Flowchart 2 below suggests a process to facilitate the needs assessment related to equipment-related consumables.
FLOWCHART 2: Establishing usage rates and requirements for equipment-related consumable items
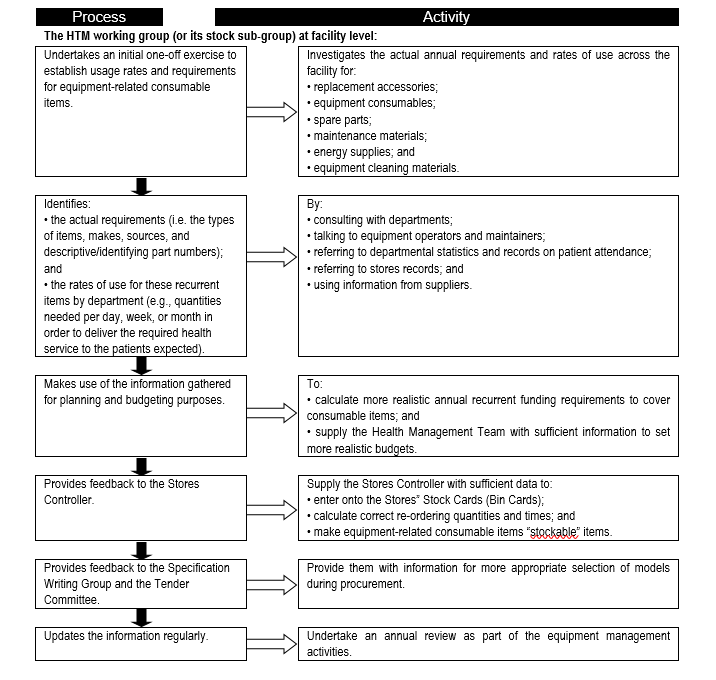
Asset management
Inventory
It is essential that an inventory (asset register) of medical equipment is maintained. However, it is both costly and time-consuming to include each and every medical device in an asset register. For those items in an asset register, the following information should be captured and verified:
- Equipment type (an international nomenclature system such as the Universal Medical Device Nomenclature System (UMDNS) should be used so that all institutions use a common name for the same type of device).
- Make/manufacturer.
- Model.
- Serial number.
- Date of acquisition.
- Price paid (include any costly accessories).
- Supplier details (name, address, contact person, contact details).
- Location – department or ward where the unit is used.
The responsibilities, activities and role-players relating to equipment inventory are shown in Box 5.
Box 5: Inventory-related responsibilities, activities and actors
| Body | Responsibility | Activity | People involved |
| HTM Service | Creates and updates the Equipment Inventory. | Organises the gathering of inventory data. | Either by:
|
| Inventory team | Visits each department in the health facility, and:
If existing records are available:
|
Due to the workload and knowledge required, it is useful for the team to be made up of:
| |
| HTM teams | Compile the Equipment Inventory.
Make hard copies. |
|
Make use of trained technical staff and secretarial/computing support to assist with data entry. |
| Central-level HTM team | Develops the Equipment Inventory as an active (regularly updated) resource. Analyses the Equipment Inventory for planning purposes. |
|
Makes use of support from staff trained in keeping computerised records. |
Asset management system
Proper asset management requires an appropriate information system which can be used to maintain data on those items that have been included in the asset register.
Equipment audits provide a snapshot of equipment status in a facility or groups of facilities, and can be aggregated to reveal the situation at national level. Audit data also serves to inform decision making related to needs assessment, asset management, maintenance strategies, replacement planning, and so on.
Also, once an inventory system is up and running, it will be necessary to periodically obtain estimates of total equipment stock values – this is used, for example, in benchmarking the total expenditure on maintenance. Flowchart 3 below shows the process for obtain such estimates.
Asset management software
It is essential to adopt and standardise on an appropriate asset management information system. There are a number of such systems available; serious consideration should be given to open-source solutions and/or or solutions which are cost-effective and sustainable, requiring minimal support.
Flowchart 3: How to estimate total equipment stock values
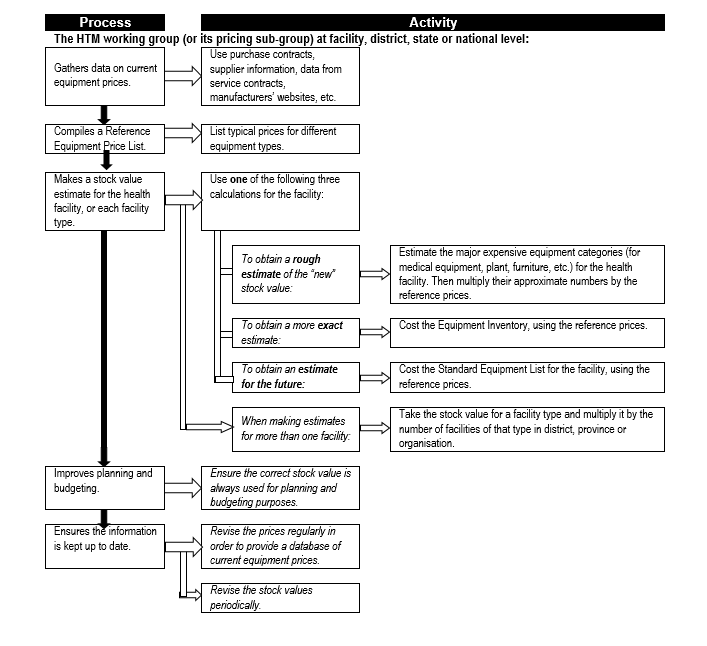
Budgeting & financing
Estimates of budget lines for equipment expenditure
It is essential to have budget lines for health technologies/medical equipment in national, province, district and facility budgets. A process for developing budget lines is shown in Box 6.
Box 6: Process for developing budget lines for equipment expenditure
| People responsible | Action |
| Finance officers, at all levels of the health service (central, province, district, facility) | Establish different budget lines (sub-divisions) as itemised below:
a. capital funds to cover equipment replacement (depreciation); b. capital funds to cover additional new equipment requirements; c. capital funds to cover support activities which ensure equipment purchases can be used (installation, commissioning, and initial training); d. capital funds to cover pre-installation work for equipment purchases; e. capital funds to cover major rehabilitation projects; f. recurrent funds to cover equipment maintenance costs, including spare parts, service contracts, and minor works; g. recurrent funds to cover equipment operational costs, including consumable items and worn out accessories; h. recurrent funds to cover equipment-related administration, including energy requirements; and i. recurrent funds to cover ongoing training requirements. |
| HTM working groups | Start using these budget lines to analyse how money is allocated and spent for equipment purposes. |
| Health service providers | Ensure that budgets are presented by cost centre so that it is clear what allocations are made between national, provincial, district and facility levels. In this way, it can be seen what money is spent on equipment activities at each level of the health service. Lobby other bodies involved (such as Ministries of Finance, Public Works) to also show equipment expenditures to establish what is allocated by other agencies for equipment activities in the health service. |
The high-level budgets thus obtained can then be “unpacked” into more detailed line expenditures, such as shown in Table 1 below, with projections covering 3-5-year budget cycles.
Estimates of maintenance costs for forward planning
An important – and often neglected – expenditure category is that of maintenance, be it for medical equipment or healthcare technologies and infrastructure in general. Box 7 unpacks the maintenance line item to indicate the various categories of maintenance-related expenditure for medical equipment, while Flowchart 4 suggests a process for estimating maintenance costs as part of planning.
Table 1: Example of a core equipment expenditure plan
| Capital expenditure | Short term | Medium term | ||
| 2012 | 2013 | 2014 | 2015 | |
| Replacement | Use calculations for rough estimations (see Note below) | |||
| New equipment | ||||
| Support activities linked to purchases | ||||
| Pre-installation | ||||
| Rehabilitation | ||||
| Sub-total | ||||
| Recurrent expenditure | ||||
| Equipment maintenance | Use calculations for rough estimations (see Note below) | |||
| Consumables | ||||
| Administration | ||||
| On-going training | ||||
| Sub-total | ||||
| Total expenditure | ||||
Note: Initially, rough estimates are used for the short- and long-term overview when preparing this Core Equipment Expenditure Plan. During annual planning the estimates are revised, using calculations for specific requirements, to obtain the Annual Equipment Budget. The experience gained from that annual revision process may mean that the long-term estimates in this Core Equipment Expenditure Plan may have to be altered, so that they are more realistic.
Box 7: Elements of annual maintenance budgets
|
I. Planned budgets: These allocate funds for anticipated maintenance costs, which can be derived from the following main areas of expenditure: a) spare parts – which are required regularly, determined from previous experience and any planned remedial work; b) spare parts – which are required according to planned preventive maintenance (PPM) schedules and timetables; c) maintenance materials – which are required regularly, determined by previous experience and any planned remedial work; d) maintenance materials – which are required according to PPM schedules and timetables; e) service contracts – required for any planned remedial work; f) service contracts – for breakdowns which are likely to be required, determined from previous experience; g) service contracts – required for PPM of complex equipment; h) calibration of workshop test equipment; i) replacement of tools at the end of their life; j) office material; and k) any increased maintenance requirements brought about by planned new equipment purchases under the capital expenditure budget. Note: there will be other elements which may fall under other budgets. These could include:
|
|
II. Contingency budgets: In addition to planned budgets, contingency budgets also exist. These allocate funds for unplanned maintenance work, such as emergencies, or sudden breakdowns which could not be predicted. |
Flowchart 4: How to make rough estimates of maintenance costs for forward planning
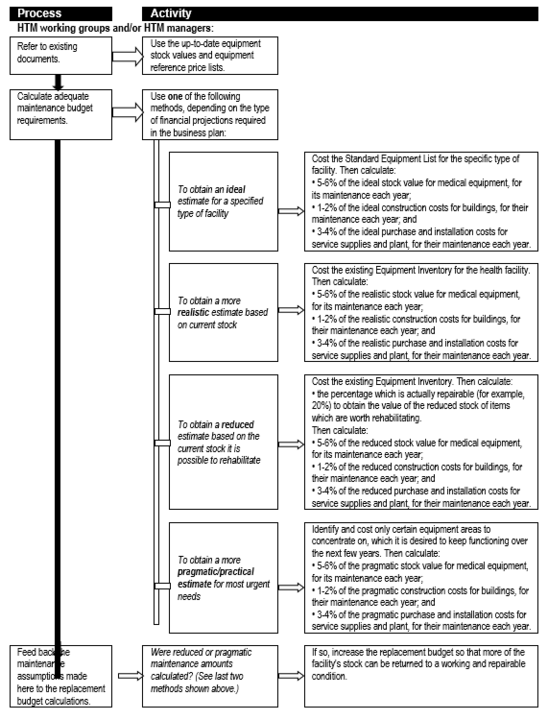
Estimates of consumable operating costs for forward planning
In Section A2 (Needs assessment) the importance of consumables for proper functioning and utilisation was highlighted. Since medical devices cover such a wide spectrum, the related consumables require insight and good management. A number of alternative approaches are suggested:
i. Consumption depends on the type of equipment used, the service provided, and how many patients are seen. Therefore, a rough estimation of consumable operating costs can be obtained by evaluating past usage rates/expenditures, and comparing these with expected patient loads and specific equipment usage rates per intervention.
ii. If the equipment is part of a “closed” purchasing system, the consumables are only made by one manufacturer and one is limited to a single supplier; this monopoly often increases the consumable costs. If the equipment is part of an “open” purchasing system, anyone can supply the consumables and different manufacturers’ consumables could be used; this competition brings down costs of consumable. Costs can also be kept down by using items which can be sterilised/re-used.
iii. Consumable operating costs vary according to equipment type, and can be expressed as a percentage of purchase cost or stock value, as shown by the examples below. But as the majority of equipment is likely to be technology that has low to medium consumable costs, one could use averages of 3% of the stock value for equipment with low consumable usage rates, and for others as shown in Table 2.
Table 2: Rough estimates of consumables’ operating costs for forward planning
| Description | Consumable cost per year (relative to original purchase cost) |
Equipment with high consumable operating costs, such as:
|
70-120% |
Equipment with medium consumable operating costs, such as:
|
20% |
|
15-25% |
|
10-15%
5-15% |
Equipment with low consumable operating costs, such as:
|
2-5% |
|
1-2% |
A different calculation is required when making specific or annual estimates. Annual operating budgets should be based on more exact estimates. These are not always easy to predict since epidemics, outbreaks, or surges in workload cannot, in most cases, be anticipated.
Generally with experience, and where standardisation of equipment is in place, the projection for equipment consumables and spare accessories becomes more predictable.
Specific or annual estimates of consumable operating costs
It is equally important to make provision for consumables on an annual basis. Flowchart 5 suggests how this could be done.
FLOWCHART 5: How to make specific or annual estimates of consumable operating costs
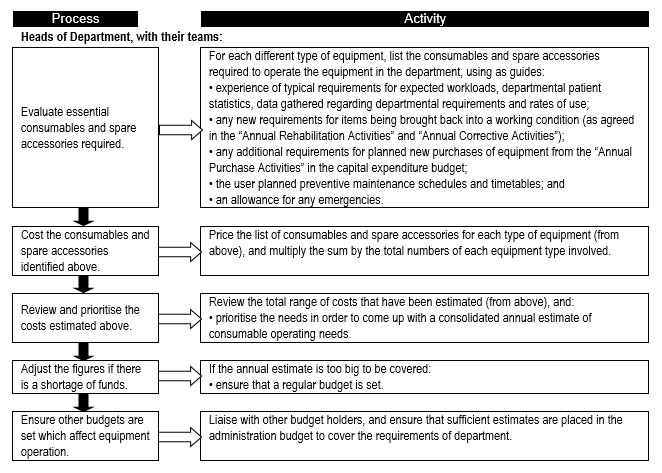
Estimates of equipment replacement costs
Equipment replacement needs to be provided for, especially in the case of complex and expensive equipment, to ensure that adequate resourcing is available at the appropriate time to ensure continuity – or at least minimal disruption – of service delivery. Flowchart 6 below suggests a process to establish estimates for replacement costs.
The administrative costs associated with medical equipment are also seldom considered separately, since they are hidden within general overheads. Different countries suggest alternative approaches:
i. Administrative costs are a small percentage of any operating budget; for example:
- the biggest percentage expense is for staff, taking 50-55%;
- supplies/spares take 35-45%; and
- administration takes only 10-20%.
Thus an equipment-user department could use an average of 15% of their its total operating budget for administrative costs.
ii. For HTM teams and clinical engineering service maintenance workshops, their administrative needs are not much higher than other administrative units in health facilities. Therefore, a reasonable estimate for the administrative costs[1] for HTM teams could be calculated by taking 10-20% of their total operating budget.
iii. A starting point is to use 5% of the equipment stock value to cover equipment-related administrative costs.
FLOWCHART 6: How to make rough estimates of replacement costs for forward planning
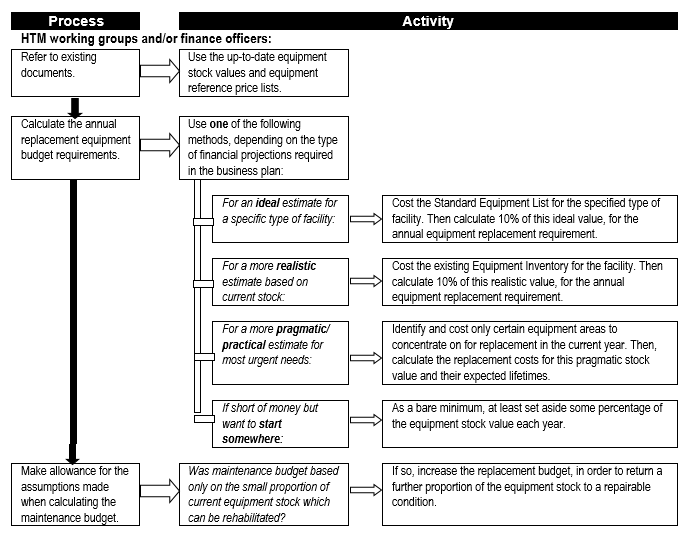
As in the case of consumable-related costs, it is important to determine the annual equipment-related administrative costs (Flowchart 7).
FLOWCHART 7: How to make specific estimates of assorted equipment-related administrative costs
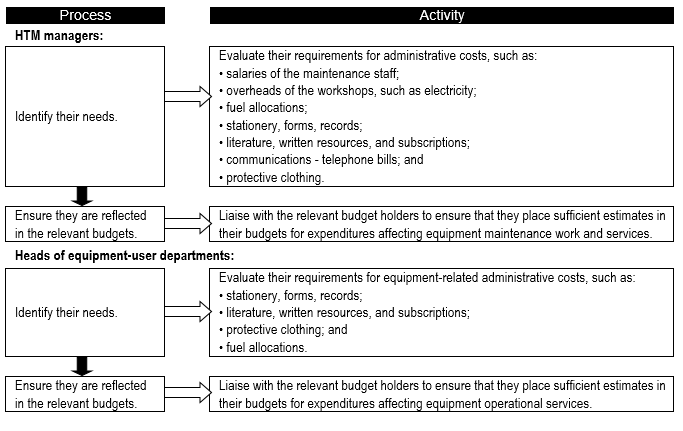
Requisitioning
The following checklists cover three commonly encountered scenarios:
Checklist 3: Request to purchase new equipment that is not yet in use at the institution
a. Why is it essential to have this equipment and how will it enhance the present patient care. How was this function performed before and up until this request?
b. Statistics of the patients who will be treated with this equipment.
c. Is this equipment in line with the norms of the service delivery – level of care –of the institution?
d. Accessibility to similar equipment or services in a close proximity to the Institution.
e. Is suitable accommodation available to install the equipment (building and facilities). Is the structure suitable to carry the additional mass? This to be confirmed by the Engineering Services Manager, in writing.
f. Are there engineering services available to operate the equipment and has the availability of the service been confirmed in writing by the Engineering Services Manager, for instance:
- sufficient water pressure and flow (hot and cold);
- sufficient water pressure and flow (hot and cold);
- medical gas and compressed air (at the correct pressures and flow);
- sufficient power at the correct voltage and current levels;
- if required, a UPS system with a sufficient capacity;
- a power line-conditioning unit for sensitive electronic equipment;
- if required (for example autoclaves), is steam, condensate return and drainage available; and
- if required, is the ventilation and air-conditioning sufficient.
g. Are proper specifications available from the HTM unit or is suitable equipment available from an approved period tender? State the tender and the item numbers.
h. Confirmation must be obtained from the Institution that they have a budget (sufficient funds) to pay for a service contract (where called for), as well as consumables and preventive and corrective maintenance of the new equipment.
i. Will not having the equipment in any way compromise patient care or safety?
Checklist 4: Request to replace existing equipment
a. Inventory of similar equipment available in the Institution.
b. Inventory of how many pieces of the type of equipment are functional.
c. Inventory of how many pieces of equipment are not functional and the reason thereof.
d. Is this equipment in line with the norms of service delivery – level of care – of the institution?
e. Reason for the condemning of the equipment, supported by a Condemning Certificate.
f. Where the replacement is a fixture for instance an x-ray machine or processor, what engineering or structural changes will be required?
g. Does the Institution have sufficient funds to purchase the equipment requested?
h. Does the Institution have a budget, sufficient funds to pay for a Comprehensive Service Contract for the equipment requested?
i. Is suitable accommodation available to install the equipment (building and facilities) and is the structure suitable to carry the additional mass? This to be confirmed by the Engineering Services Manager, in writing.
j. Are there engineering services available to operate the equipment and has the availability of the service been confirmed in writing by the Engineering Services Manager, for instance (as for above scenario – Checklist 5)?
k. Can this equipment be standardised for the reasons previously stated above?
l. Statistics of patients to be treated with the equipment requested.
m. Are there no other procedures available to treat the patients?
n. Will not having the equipment in any way compromise patient care or safety?
o. Are there proper specifications from the Health Technology Unit available, or is suitable equipment available from an approved period tender? State the tender and the item numbers.
Checklist 5: Request to purchase additional equipment similar to equipment already in use
a. Inventory of similar equipment available in the Institution.
b. Inventory of how many pieces of the type of equipment are functional.
c. Inventory of how many pieces of the type of equipment are not functional and the reason therefore.
d. Is this equipment in line with the norms of service delivery – level of care - of the institution?
e. Reason for requesting the additional equipment backed up with patient statistics. Has the function or status of the Institution changed?
f. Does the institution have sufficient funds to purchase the equipment?
g. Does the institution have a budget, sufficient funds to pay for a comprehensive service contract for the equipment requested?
h. Can this equipment be standardised for the reasons previously stated?
i. Are there no other procedures available to treat the patients?
j. Will not having the equipment in any way compromise patient care or safety? Are there proper specifications available from the Health Technology Unit or is suitable equipment available from an approved period tender. State the tender and item numbers?
Procurement
Introduction
Procurement, from a healthcare service perspective, is probably the most important activity within the medical equipment life-cycle. If the right (competent) people are driving a transparent, criteria-driven (not interest-driven) process, it is likely that the healthcare system will avail itself of the technology that is most appropriate to the intended application, while addressing issues around effectiveness, cost-of-ownership, institutional fit, technology maturity and compatibility, user competence, maintenance and so on. In many countries, procurement is being placed in the hands of generic supply-chain management personnel with little specialised knowledge of medical equipment specifically, and health technologies in general. It is therefore essential to ensure that a supporting environment is created to minimise the possibility of sub-optimal procurement outcomes.
Procurement (for capital equipment as opposed to consumables) should be seen as a process with two phases: one that commences with planning and needs assessment and ends with commissioning, and the other that extends over its operational lifetime and ensures that the equipment is provided with the necessary accessories, consumables, maintenance support, and so on.
There are four reasons for procuring equipment, each of which provides a different goal which will dictate when to acquire equipment. These can be placed in the following order of priority (see Box 8 below). Within each of the four categories shown, priorities will have to be set, and these can be based on appropriate indicators.
Box 8: Example of valid reasons and order of priority for purchasing of equipment
| 1. To cover depreciation of equipment. | Equipment is replaced as it reaches the end of its life and is taken out of service. This is necessary in order for the level of healthcare that is currently delivered to be sustained. [Note: This means that the size of the existing equipment stock remains the same, and does not imply an expansion of the health service.] |
| 2. To obtain additional equipment items which are missing from the basic standard requirements. | Additional equipment may be required in order to provide a basic standard level of care. [Note: Missing items are identified by comparing the Equipment Inventory with the Standard Equipment List for the facility.] |
| 3. To obtain additional equipment items beyond the basic standard. | This is done in order to upgrade the level of health service provided by the hospital. For example, new equipment may be needed to provide a new service, build a new special unit, or increase the level of care offered. |
| 4. To obtain additional equipment items outside the facility’s own plans. | This will only be applicable if the additional items have been called for by directives from the national or provincial ministry, and cannot be stopped/refused for political reasons, such as “out of the ordinary”, high profile, or political projects. |
Of course, procurement is not a self-contained, isolated process but links up with many other equipment-related processes; these are shown in Box 9 below.
Box 9: Planning tools that assist in deciding on procurement
| Planning tools | How they help |
| When replacing items: | |
| Replacement policy | Establishing and implementing this tool is much more likely to ensure that the necessary regular planned replacement of equipment takes place. |
| Equipment inventory and maintenance record system | These tools support identification of the need for replacement equipment at any time. For easy reference, estimated lifetimes for equipment could be entered into the inventory and could then prompt one when to purchase. The natural life of equipment is shortened by harsh environment, over-use, unskilled handling, neglect of maintenance and damage. Equipment malfunction and downtime also increase with the age of the equipment. Accordingly, cost-effectiveness decreases with age. |
| Replacement and condemnation criteria | These assist with judging when equipment has reached the end of its life, and therefore with identifying when equipment needs replacing. |
| Core equipment expenditure plan | Some of the equipment will have to be replaced every year, so it makes sense to spread budgeting for replacement over time. By allocating some money each year, one can avoid facing a large replacement bill later on. This tool should spread these costs over the long term. Equipment replacement needs can be estimated each year by assuming that each piece of equipment has an average lifetime of 10 years [1] - this means that on average 10% of equipment stock needs to be replaced each year. |
| Disposal policy and disposal procedures | When equipment is replaced, these tools will assist with the disposal of the old device or system. There may be government regulations regarding disposal that will provide additional information. |
| Stock- control system | Replacement equipment-related supplies should be purchased after an up-to-date stock take. Accurate stock control systems help with planning and ordering. |
| When buying additional new items: | |
| Equipment development plan, and annual purchase plan | New equipment should be purchased according to an annual purchase plan (drawn each year from a long-term equipment development plan). This is based on an up-to-date inventory and Standard Equipment List. Accurate inventory record-keeping helps with planning and ordering. |
| Package of inputs | Planning in advance for what will be needed after the purchase is every bit as important as the purchase itself. Thus, one must ensure that the package of inputs required to keep equipment functioning through its life is procured. |
| Core equipment financing plan, and annual budget | Capital expenditure can only take place once sufficient funding sources have been identified. The long-term Core Equipment Financing Plan and Annual Budget should allocate known and possible sources of funds against elements of planned expenditure. |
Additional items of equipment may need to be procured to accompany/complement the original item, if it is to function properly in certain environments. For example:
- a voltage stabiliser (surge suppressor plus filter) - this offers protection against power supply fluctuations, but does not protect against power cuts. It monitors the power supply, removes surges and spikes, and maintains a continuously regulated alternating current output to the item;
- an uninterruptible power supply - this offers protection against blackouts and power cuts of limited duration;
- an air-conditioning unit; and
- a water filter or treatment plant.
Preparation of specifications
Specifications have to be drawn up for every device that is planned to be purchased. Standardised specifications need to be drawn up for commonly used devices which can then be modified (where necessary) at institutional level.
General terms and conditions should also be part of specifications, including stipulations like the local availability of essential spare parts, and the presence of a registered sole agent for the specific brand.
Specifications should be functional specifications and drawn up based on features available in at least a few brands of the device commonly available in South Africa. Before making a selection the following may be considered:
- demonstration of devices by supplier;
- trial use of the device in a facility;
- visit to a facility where the device is available;
- verbal presentation on device made by a supplier of the device; and
- communication with existing users of the device both locally and abroad.
A suggested format for specifications is as follows:
- name of equipment;
- Function;
- essential features;
- essential components;
- additional components;
- power supply;
- additional requirements; and
- training – user training, maintenance training.
In order to eliminate the possibility of outdated specifications being used, all specifications should have a validity date on the document. Specifications that are not valid or have expired should not be used. Copies of the latest specifications should be obtained from the appropriate HTM unit.
A sample specification (that for an infant incubator) is given in Annex I. For some equipment, such as sophisticated or imported items, or equipment which is new in the system, it may be necessary to specify the following item lines:
- Site preparation details – supplier should provide technical instructions and details so that this work can be planned, either in-house or by contracting out.
- Installation – assistance may be needed.
- Commissioning – assistance may again be required.
- Acceptance – the responsibilities of both the purchaser and supplier with respect to testing and/or acceptance of the goods must be clearly detailed.
- Training of both users and technicians – help must be obtained if required.
- Maintenance contract (an important part of after-sales support) – help must be requested if it is required. It will be necessary to agree and stipulate the duration, and whether it should extend beyond the warranty period, the cost and whether it includes the price of labour and spare parts, and the responsibilities of the owner and supplier.
There are a number of technical and environmental factors that need to be taken into account. For example:
- If the area has an unstable power supply, is the supplier able to offer technical solutions (such as voltage stabilisers, an uninterruptible power supply)?
- Will the geographical location (such as height above sea-level) affect the operation of equipment (such as motors, pressure vessels)? If so, can the manufacturer adjust the item’s specific needs?
- Extremes of temperature, humidity, and dust may adversely affect equipment operation, and may require solutions such as air-conditioning, silica gel, polymerised coatings for printed circuit boards, and filters.
This information can be included within the generic equipment specifications. However, since much of the information is common to many pieces of equipment, some health service providers have found it simpler to develop a separate summary Technical and Environmental Data Sheet, which can be referred to in the purchase documents. This data sheet can be distributed to all suppliers, interested parties, trade delegations and other relevant bodies. Such a data sheet can be provided regardless of the length of specification or the procurement method used, ensuring that all parties are kept informed of prevailing national conditions which could affect the operation of equipment.
The following details should be included in a Technical and Environmental Data Sheet:
- Electricity supply – mains or other supply, voltage and frequency values and fluctuations.
- Water supply – mains or other supply, quality and pressure.
- Environment: height above sea-level; mean temperature and fluctuations; humidity; dust level; vermin problems, etc.
- Manufacturing quality – international or local standards required.
- Language required – main and secondary.
- Technology level required – manual, electro-mechanical or micro-processor controlled.
Evaluation and comparison process
The process of evaluation and comparison can often be time consuming. However, it is important to ensure that decisions for awarding contracts are not made simply on the basis of which items are the cheapest. During evaluation the items should be assessed against the requirements specified in the purchase document. The most common way to evaluate offers is to use an elimination process, where some offers are rejected at each stage. Offers should be judged against the following criteria:
- Compliance with requirements in the purchase document.
- Technical nature of the offer (part of the product selection criteria).
- Financial nature of the offer (part of the product selection criteria).
- Supplier qualification criteria.
By doing this, decisions are based on best value for money for the whole life-cycle cost, rather than simply being based on the item’s purchase price.
The process of evaluation and comparison must be fair and thorough. To achieve this, the process must follow a defined pattern to ensure all bids/quotes are dealt with in exactly the same way.
The evaluation process for tenders is similar to that for quotes, although the tender process is normally a far more comprehensive task and is also regulated by law. The tools for evaluation can be the same, but the amount of information required is usually much less with quotation methods. Obviously, if only three quotes are requested for a simple small order, the evaluation process should not take much time. The following evaluation steps are used, and some bids/quotes are rejected at the end of each step.
Step 1: Checking for compliance
The first step when evaluating offers is to determine which, if any, are not compliant with the technical, commercial, and other specifications in the purchase document. The procurement unit should be able to do this. It involves a more detailed examination of the offer to determine the compliance by the bidder with the requirements specified in the purchase document. This is known in tender processes as the substantial responsiveness of the bid. This process is more formal and comprehensive for tenders than for quotations.
A substantially responsive bid is one which:
- conforms to all the terms and conditions; this means that the supplier has responded to all parts of the schedule of requirements, has filled in all the boxes, and is able to supply all the parts required; and
- also establishes the bidder’s qualifications to supply and deliver the products within the delivery schedule; for example, the supplier:
- has enclosed signed audited accounts, signed company declarations on health, safety, and environmental activities, certificates of quality manufacturing, and a letter of authorisation from the manufacturer, and
- has nominated a representative in the country (if this was one of the compliance criteria).
All non-substantial bids will be rejected as non-responsive and should be excluded from further in-depth evaluation.
In practice it is found that most bids contain reservations from one or more of the detailed requirements. If these are just minor adjustments and do not represent a substantial deviation from expressed interests, one can still conclude that the bid is substantially responsive and therefore should be included. However, the reservations for closer clarification should be listed.
To assist in this examination one could ask for other clarifications from the bidder. One may also wish to inspect the quality methods and production methods stated in their documents. This request and response should be in writing. The bidders, however, are not allowed to make any changes to the substance or price of their offers. After evaluating the offers for compliance, some offers are discarded as the suppliers fail. The remaining offers can go forward to be compared in terms of technical performance (Step 3).
Step 2: Preparing the evaluation information
The procurement unit should collate and compile the information from all the responsive bids into evaluation information sheets. Box 10 gives an example of the sort of data that needs to be included.
Box 10: Evaluation information sheets
| Evaluation Information Sheets present an easy way to compare different bids/quotes. For ease of comparison, it is best to allocate one page per subject matter (technical product details, price, etc.) and compare the offers side-by-side. Areas where each offer deviates from the equipment specification should be highlighted. Typical evaluation information sheets would include the following evaluation criteria:
Accessories, consumables, spare parts:
After-sales service:
Price information:
Note: this is an example and not a comprehensive list. The information chosen to include will depend upon the complexity of the equipment purchased – less technical detail is needed for simple equipment or recurrent supplies. |
Ideally the evaluation information sheets should allow side-by-side comparison of the offers. For tenders there are more responses and the orders tend to be more complex, so more paperwork is required to obtain a true comparison. Usually there is too much information to be compiled in a single sheet, so it may be better to use:
- one sheet for collating purely technical information about the product;
- one for information on accessories, consumables, and spare parts;
- one for after-sales service; and
- one for price information.
The procurement unit should present the evaluation information sheets to the procurement/tender committee (for tenders and high-value quotes) or the Procurement Manager (for low-value quotes). They should be accompanied by an explanation of the requirements, main objectives, and any binding constraints. Then the technical evaluation process (Step 3) can commence.
Step 3: Carrying out the technical evaluation
The procurement/tender committee should base its decision on advice from members of the group with professional knowledge relevant to the offer. If appropriate, it should also seek advice from representatives from the relevant user department and HTM team (for low-value quotes, the Procurement Manager should also seek advice from these end-users).
The committee should consider the product selection criteria, as set out in the purchase document. It is essential that the information provided by the supplier is related only to the equipment specification and the selection criteria listed in the purchase document – any additional information intended to sway the evaluation can be seen as corruption; hence the importance of detailing requirements adequately in the purchase document. The better the purchase document, the easier the evaluation process will be. Box 11 lists a few points to remember, relating to the technical aspects of the offer.
Box 11: Summary technical assessment
|
For both quotations and tenders it may be necessary to ask the supplier to clarify any ambiguities or uncertainties. Alternatively, a shortlist of suppliers can be drawn up and those included can be asked to demonstrate their equipment, as part of the technical evaluation (this is unlikely to be necessary for very simple equipment, and may be impossible for many overseas suppliers).
After the technical evaluation, some offers are discarded if they fail to meet the requirements. The remaining offers can go forward to be compared in terms of financial performance.
Issues to consider when choosing equipment
Choosing equipment is not easy, due to the wide range of products available. External influences also play a part. For instance, external support agencies may impose their own conditions regarding suppliers, which may result in inappropriate equipment being supplied or procured. The acquisition policy should clearly specify the “good selection criteria” to employ. All equipment should:
- be appropriate to its intended target setting;
- be of assured quality and safety;
- be affordable and cost-effective;
- be easily used and maintained; and
- conform to the existing policies, plans and guidelines.
These criteria, unpacked in Checklist 6 below, should be used during the procurement process when evaluating and adjudicating between different offers from suppliers.
Checklist 6: Example of good selection criteria for equipment purchasing
| Indicators of appropriateness | Criteria |
| Appropriate to setting | Equipment should be:
|
| Assured quality and safety | Equipment should be:
|
| Affordable and cost-effective | Equipment should be:
|
| Ease of use and maintenance | Equipment should be chosen
|
| Conforms to existing policies, plans and guidelines | Equipment should be chosen:
|
It may also be appropriate to have a set of criteria to evaluate equipment suppliers, both current and past, as per Checklist 7.
Checklist 7: Suggested criteria for evaluating current and past suppliers
| Issue | Criteria |
| Participation record |
|
| Response to enquiry |
|
| Delivery time (for consumables) |
|
| Adherence to delivery instructions |
|
| Provisions of documents |
|
| Packing and labelling (mainly for consumables) |
|
| Product shelf life (for consumables) |
|
| Compliance with contract financial terms |
|
| Quality of products and services |
|
Adapted from: Management Sciences for Health, 2002, “Managing drug supply”, MSH, Boston, USA.
Donations
Donations are a special case of procurement, and great care needs to be exercised in managing the procurement process associated with donated equipment. The World Health Organization (WHO)[1] and other bodies have drafted guidelines to facilitate this process.
Receipt, testing, installation & commissioning
Pre-installation work / site preparation
The site at which the device is to be installed has to be adequately prepared, more so in the case of large, sophisticated devices like x-rays, autoclaves. This has to be coordinated with logistics of the device supply so that the site is ready when the device is delivered at the institution. The associated utilities like appropriate power supply, water, compressed air and the like, as well as other appropriate requirements like radiation protection should also be taken into consideration. In situations where extensive site preparation is necessary, like in x-rays or CT scans, site works should be included as part of the acquisition process to ensure comprehensive and coordinated site preparation.
Pre-installation work involves:
- preparing the site ready for equipment when it arrives;
- organising any lifting equipment;
- organising any warehouse (storage) space;
- confirming installation and commissioning details; and
- confirming training details.
In some cases, the pre-installation work required is minimal; in others it requires considerable labour and finance. As a general guide, site preparation is the work required to ensure that the room or space where the equipment will be installed is suitable. It often requires the provision of new service supply connections (for electricity, water, drainage, gas, waste) and may require some construction work. Site preparation tasks can include:
- disposing of the existing obsolete item (disconnection, removal, cannibalising for parts, transport, decontamination and disposal);
- extending pipelines and supply connections to the site, from the existing service installations;
- upgrading the type of supply, such as increasing the voltage, or the pipeline diameter;
- providing new surfaces, such as laying concrete, or providing new worktops; and
- creating the correct installation site – for example, digging trenches, building a transformer house or a compressor housing.
In considering where to position equipment, the following types of questions should be asked:
- Is there sufficient access to the room/space? (Door sizes and elevator capacity are very important for x-ray and other large machines.)
- Is the room/space large enough?
- Is the position and layout of the room/space suitable?
- Are the required work surfaces and service supply points available?
- Is the environment adequate for the purpose? (For example, is it air-conditioned? Dust-free? Away from running water?)
If new buildings or extensions are being constructed, different relevant departments and groups need to work closely together to design the rooms and plan the service supplies. Planners, users, architects, service engineers, and equipment engineers need to be consulted.
Site preparation can be carried out by:
- in-house staff (for example, the facility HTM team or a central/regional HTM team);
- maintenance staff from other national agencies (for example, electricians from the Ministry of Public Works);
- a contractor who has been hired (for example, a private company or an NGO partner); and
- the suppliers or their representative.
When planning and budgeting for the equipment, site-preparation costs should have been estimated for inclusion in the budget. However, as soon as the order is placed, the HTM working group / procurement unit should provide the supplier with details of the proposed equipment site and services, and officially request the necessary site preparation instructions.
Once these are received the HTM manager can plan the work, quantify the needs and costs for materials and contractors, and apply for a budget allocation. He or she should then oversee the work, and ensure it is undertaken before the equipment arrives.
Flowchart 8 below shows the common site preparation steps that may be required, depending on the type of equipment purchased. By the time the goods start to arrive, the site should be ready to receive them.
FLOWCHART 8: Common site preparation steps
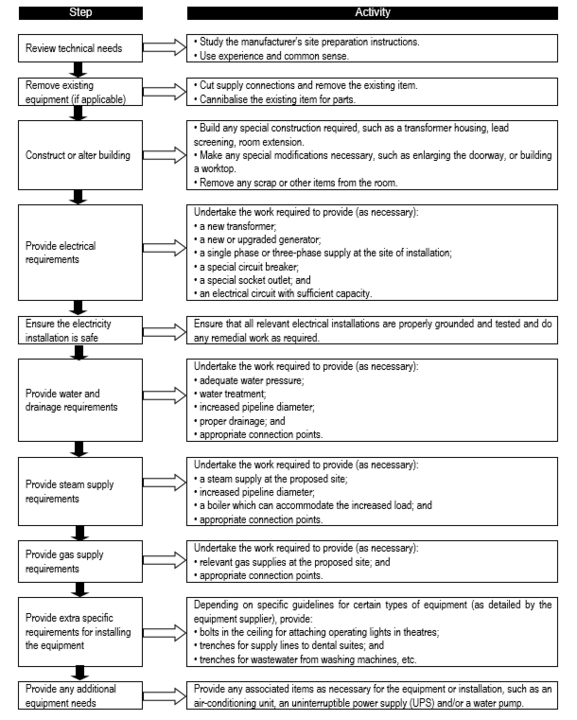
An overview of the acceptance process
Each health facility should have an official acceptance process for equipment that arrives on site (a simpler process is used when equipment-related supplies arrive on their own). During the acceptance process, the following should be established:
the complete order has arrived;
- installation, commissioning, and initial training has taken place;
- the equipment is mechanically and electrically safe for users and patients and is functioning properly; and
- the equipment is entered into the health facility inventory.
A simple way to carry out these activities is to fill in a standard Acceptance Test Logsheet. This form is specially designed to make checking easier and to help to avoid mistakes. It is an important document since it is the first record to be placed in the equipment file and provides all relevant details of the start of the equipment’s life at the health facility, and commences the service history of the equipment. The Acceptance Test Logsheet has sections that cover all the components of the acceptance process, including:
- delivery/receipt of the equipment on site;
- unpacking and checking for damage and for the complete order;
- assembly;
- installation;
- commissioning and safety testing;
- official acceptance;
- initial training;
- registration – entering stocks into stores and onto records; and
- handover.
Each of these sections in the logsheet needs to be completed and signed off to indicate that the activity has been successfully completed. Once the logsheet has been fully completed, it is signed off to certify that the equipment and services are satisfactory. Only then should payment be made.
If there are problems with goods or services, the Acceptance Test Logsheet should not be signed; instead write a fault report on the equipment’s shortcomings should be logged, outlining the problems encountered and advising that payment be withheld until the problems have been addressed.
The equipment is not normally put into routine use until the complaints have been resolved, the logsheet finally signed off, and the payments made. The acceptance process is straightforward for common low-complexity items of equipment that are simple to use. Installation, commissioning, and initial training are not major activities and can happen all at once – e.g. for a mobile examination lamp:
- Installation involves using a test meter to check the electricity supply of the socket outlet, and then simply plugging in the lamp.
- Commissioning involves using a test meter to check the electrical safety of the lamp so that it will not give the operator an electric shock.
- Initial training involves ensuring the operator knows where the on/off switch is, how to handle the light bulb, and how to alter the angle of the head without pulling the lamp over.
Commissioning
Commissioning usually, but not always, takes place straight after installation. The technical and safety aspects of the equipment should already have been specified and considered during the selection process. However, it is also essential to carry out performance and safety tests on each piece of equipment. Such tests validate that each piece of equipment is safe and is capable of performing its intended function. Performance and safety tests should be carried out regardless of whether equipment is purchased, donated, leased or borrowed by the health facility. The commissioning team and any visiting installers are responsible for ensuring these tests take place. The steps in the commissioning process are shown in Flowchart 9.
FLOWCHART 9: Key steps in the commissioning process
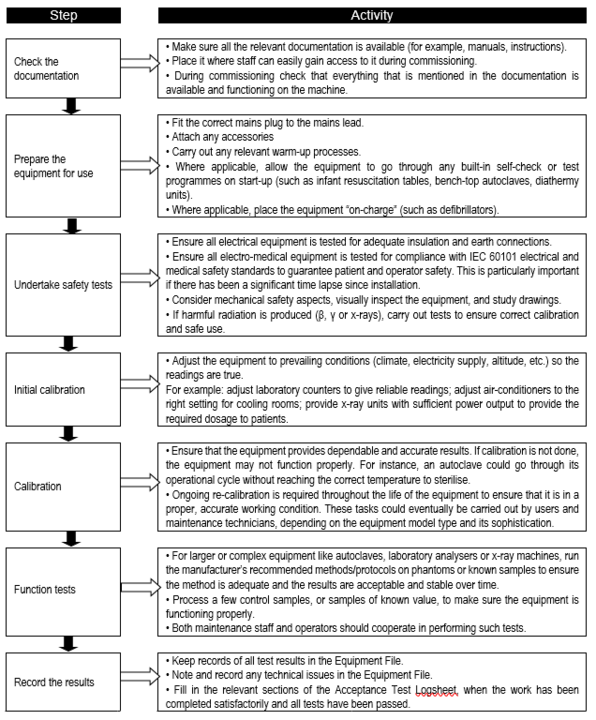
Once the equipment has passed its safety, calibration, and function tests, the commissioning team is in a position to:
- officially accept that the equipment has been received in a satisfactory condition, and
- officially accept the equipment as the institution/department’s property.
This could trigger the payment for the goods only. Payment for services can only occur when the training is finished, if it was part of the purchase contract. If the equipment has not passed the tests, negotiations would be commenced with the supplier and complaints procedures initiated. The equipment should not be accepted or used until these issues have been resolved. Once the equipment is accepted, staff can be trained in its operation and maintenance.
Registration and handover
Once equipment and supplies have been officially accepted, they can be registered in various health facility records and systems, process them, and stored or used as appropriate. The commissioning team is responsible for ensuring that all these activities take place.
Entering new equipment orders into health facility records
All equipment and equipment-related supplies need to be entered into the health facility’s records and systems. The most common records and systems are the:
- Equipment Inventory (manual or computerised). The HTM manager should enter new major pieces of equipment onto the Equipment Inventory. The type of information recorded should identify the particular piece of equipment, its manufacturer and location. The HTM manager can gather this information from the Acceptance Test Logsheet and the Register of New Stocks form. In addition, the HTM manager should allocate a unique inventory code number to each piece of equipment and ensure the equipment is labelled/marked with this code number.
- Equipment File (manual or computerised). This acts as a service history for a particular piece of equipment. The HTM manager should open a new Equipment File for each piece of equipment, and label the file with the equipment’s inventory code number. The type of information recorded in this file at the acceptance stage should include details of the manufacturer/supplier and purchase contract terms. Much of this information will be contained in the completed Acceptance Test Logsheet, which should be the first document placed in this file with supplementary data as required. Subsequent records placed in the Equipment File are of any maintenance work carried out so that the file becomes the equipment’s service history.
- Planned preventive maintenance (PPM) programme. The HTM manager should register equipment for any PPM carried out by maintenance staff (if it is taking place), and enter it onto their PPM timetable so that it gets attention at regular intervals. On handover of the equipment to the user, the HTM manager should liaise with the head of the user department about registering the equipment for any user PPM [BvR1] taking place, and entering it onto their user PPM timetable [see Section 10.2 for further discussion on maintenance].
- Equipment card. This is a piece of card or laminated sheet that is permanently kept with the equipment. It can provide users with a summary of the equipment care instructions and a summary service history, such as dates when routine inspections, testing, and servicing took place.
- Register of New Stocks form. This provides the Stores Controller with all the information required for entering each new piece of equipment and its supplies into the stores stock control system. The commissioning team should (partially) complete this form by gathering information from the available contract, packing lists, or invoice, according to a standard format. The type of information should identify the manufacturer/supplier’s order codes, description, and batch sizes of the equipment, consumables, accessories, and spare parts. The Stores Controller finishes completing the form by allocating and recording each item’s unique stores code. The Stores Controller provides copies of the finalised forms to the user departments and HTM team so they can order the correct replacement items in future. The HTM manager keeps this form in the relevant Equipment File.
- Registering warranties. The guarantee or warranty of the equipment (if applicable) needs to be registered. The date of commencement and the period of the warranty which have been agreed with the supplier must be entered into all relevant procurement, finance, and maintenance records.
- Note: Once the equipment has been checked and is confirmed as safe and ready for use, it is sensible to highlight this fact. Use a simple strip of tape placed across the main controls of the apparatus to clearly show that it has been tested. It is preferable to use printed warning notice tape, but other types could be used.
Storing manuals
Operator and service manuals should be supplied with the equipment, according to the purchase document. It is important to make sure that manuals are kept in a safe place, and are not lost by staff. It is also important to make them widely available for use among users and maintenance staff. This can be done by:
- Storing the original copies in a safe place, such as the clinical engineering workshop library, the main stores office, or the HTM service library.
- Making two photocopies of all operator manuals received, and giving one set to the head of the relevant user department, and the other to the HTM team or workshop responsible for the equipment’s maintenance.
- Making one photocopy of the service manuals received, and giving it to the HTM team or workshop responsible for the equipment’s maintenance.
- Asking for manuals or their content in other formats, such as CD-Rom, video, DVD.
- Scanning the printed documents into a computer to convert them into electronic copies, and making them easily available to maintenance staff at many locations.
- Recording in the Equipment File how the manuals were distributed. This helps with monitoring and updating the manuals in future.
Training
Introduction
After successful installation and commissioning, the users and maintainers need training on the type of equipment and the model purchased. This training can take place straight after commissioning, with the installation team acting as the trainers. However, the training team often involves different people, such as those with clinical or training skills. In this case, the training may take place sometime later when the training team has assembled.
Ideally, the initial training occurs straight after commissioning. After the training, the equipment can be handed over to the user department for regular use. However, if there is a delay before training can take place, one may have to consider whether to hand over the equipment before training staff. We recognise that there will be pressure to do this as all staff want to start using new equipment as soon as possible.
However, this should only be done if:
- the equipment is a type that has been used before;
- the staff are familiar with the equipment; and
- experienced staff members are in charge of its use, until the remaining staff can be trained.
Note: Anyone using or working on equipment without proper training or authority whose actions result in an accident is likely to be found negligent.
Depending on the complexity of the equipment and the staff’s previous experience of it, initial training can include:
- good practice when handling the equipment;
- basic “dos and don’ts”;
- how to operate the equipment (along with familiarisation with the symbols and markings on the machine);
- the correct application of the equipment;
- care, cleaning, and decontamination;
- safety procedures;
- planned preventive maintenance (PPM) for users; and
- PPM and repair for maintainers.
The level and nature of training provided depends on whether the equipment is:
- A standard make and model. If staff are familiar with the equipment, in-house staff could train new users and provide refresher training for other staff. The training sub-group should produce suitable and necessary training resources and handouts for the trainees, using the operator and service manuals, and any videos available.
- A new make or model. If the equipment is unfamiliar, training should be carried out by the supplier or their representative, or by a central training team with knowledge of the equipment. The training sub-group should observe the training session, obtain copies of any overheads or handouts used, and compile its own training pack for future training.
Sometimes in-depth training is given only to very few staff members who, at a later stage, will train the rest of the users. In this case, a training timetable is needed to ensure the ongoing training occurs.
Note:
- One should ensure that the chosen trainees turn up for the training sessions, and maintain records of the training that individual staff members have received.
- A library of the training resources developed should be established.
- The representatives of both the commissioning team and visiting training team should sign the Acceptance Test Logsheet, to avoid later disputes.
Correct application
Staff may feel confident about how to operate equipment, but it is imperative that they also know the correct “application” for the equipment. Staff need to be able to apply their taught (clinical) procedures correctly, and to employ the correct methods of application so that equipment is used to its fullest capacity.
Staff will need to be trained in order to fully appreciate when and how to use equipment. They will need to know:
- when different features will be employed for different patients or uses the range of assistance a machine can offer them;
- how to alter the relationship between the machine and the patient, or sample, for different purposes; and
- the different procedures to pursue for different disorders or treatments.
They will also need to understand the safety precautions they must take. Traditionally, colleges offering basic training for health are responsible for teaching clinical procedures. Thus, they must have access to the necessary equipment for this purpose, both in their teaching rooms and at suitable clinical locations such as hospitals.
It is important to note that training is an ongoing endeavour. Staff come and go, and even staff who have received initial training on an item of equipment may need refresher training. It is therefore useful to have a mechanism that will trigger training interventions. Flowchart 10 suggests appropriate triggers.
Flowchart 10: Example of prompts showing that training is required

When the device is removed from service, professional users should know how to clean it and to organise decontamination.
Special case: for home-based care or patient-operated devices
Professional users, clinical supervisors and prescribers need to make sure that training for end-users enables them to use a medical device/equipment safely and effectively, and to perform routine maintenance as appropriate for the medical device/equipment. For example, end-users of ambulatory infusion pumps should be aware of how the medical device works, including special features such as bolus delivery, and the risks of siphoning if a syringe is removed from a driver. It is also essential that end-users are provided with information contained in manufacturer’s instructions and that it is explained and, where necessary, expanded upon. Where possible, user organisations should provide the same standards of equipment and training for end-users as they do for staff.
Operation
Introduction
“Operation” of equipment means using the correct physical methods to get the equipment to work. In order to do this successfully, the user needs to know:
- the specific operating characteristics of the equipment;
- the operational procedures that make the machine work;
- how to use its various functions;
- how to make it perform its customary cycles and routines; and
- how to change the bulb, paper roll, batteries, etc.
The equipment manufacturer’s user manual is often the best source of this information. Other sources include:
- written resources from staff training sessions;
- experienced colleagues; and
- a wide range of reference material.
Box 12 provides some examples of general strategies when operating equipment
Box 12. General strategies when operating equipment (for users)
|
Each type of equipment has specific operating instructions. When operating equipment, it is also essential to follow good strategies for using consumable materials.
Equipment users should only operate equipment for which they are suitably qualified. Clinical meetings, committee meetings, departmental meetings, or specifically organised training sessions can all be used to provide application training (as appropriate).
For specialised devices e.g. autoclave, x-rays, the right type of personnel are required to operate these devices. Certification may be a requirement for some specialised devices. Regular monitoring has to be carried out for certain specialised devices (e.g. radiation safety monitoring for x-ray machines). User manuals should be provided for all devices to enable users to operate all devices safely and effectively, as well as for trouble-shooting for simple problems encountered with devices.
Care and cleaning
To maximise the life of equipment, it is necessary that equipment users and maintainers know how to look after the equipment and clean it. Users must care for and clean equipment regularly, to a given timetable. It is beneficial to do this because:
- it is easier to see faults (such as damaged suction pump tubing) when the equipment is clean;
- it prolongs the life of equipment (for example, protecting electronic parts from damage by dust, protecting metal from corrosion by liquids or chemicals, protecting rubber seals from degradation by greases);
- it protects the operator and patient from infections (from microscope eyepieces, for example); and
- it improves the performance of equipment (clean probes for ultrasound, clean seals on fridge doors, etc.).
The best information regarding the care and cleaning of equipment is usually contained in the manufacturer’s user manual and/or service manual. In addition, staff should have written resources from their training sessions and, in some cases, posters which provide the guidance and experience of their colleagues.
Internationally, the term “infection control” has come to mean control of a wide range of practices, processes and procedures in the clinical work of the health facility as a whole. Proper infection control (relating to equipment) can be achieved by making decisions about a number of different issues:
- decontamination, through appropriate use of cleaning, disinfection, and sterilisation methods, as well as monitoring sterility;
- linen handling; and
- ensuring the workplace is clean.
Information and advice must be sought from the relevant national body that is responsible for infection control, since national policies should have been established to ensure that risks are reduced. It is suggested that the central HTM working group, or its smaller sub-group, the infection control committee, should be responsible for establishing policies and guidelines to prevent contamination through exposure to blood, body fluids, body parts, and infectious agents.
Staff accountability
Finally, the issue of staff accountability should be addressed. Checklist 8 includes checks for different role-players as part of a systemic approach.
Checklist 8: Strategies for making staff more accountable
| Type of Staff | Strategies |
| Heads of section | - given overall responsibility for the equipment in their section, according to their inventory and undertake regular inventory checks to look for missing equipment and accessories;
- made responsible for conforming to the local security regulations for the facility and its site; and - ensure their staff have sufficient skills to operate and care for equipment correctly and safely, and help them to access appropriate training or reference materials. |
| Operators-in-charge | - uring every shift, made responsible for undertaking functional checks on the equipment as part of user planned preventive maintenance (PPM) schedules. |
| Equipment users | - made responsible for the state of the equipment, accessories and consumables they handle and use;
- take personal responsibility for ensuring they operate equipment correctly, and that they know the correct operating techniques and applications; - take personal responsibility for using the correct consumables in a non-wasteful way; - take personal responsibility for ensuring they operate equipment safely, and knowing the proper safety procedures; - made responsible for the daily care and cleaning of the equipment they use with the correct cleaning chemicals; - made responsible for monitoring that equipment is functioning properly and is providing the type of results expected, and reporting any faults immediately to the HTM team through their head of section; - ensure they have been specifically trained to undertake these tasks, and if they require further skill-development put in a request to their head of section; and - take responsibility for conforming to the local security regulations for the facility and its site. |
| purchasing and supplies officers | - made responsible for purchasing the correct consumables and cleaning chemicals. |
| Stores controllers | - made responsible for keeping track of stocks of consumables and materials in the various stores. |
| Health management teams | - develop a suitable working environment where managers are seen to be present, and performing well themselves;
- consider positive strategies with bonuses and rewards for good behaviour with equipment, as incentives for making staff more responsible and accountable; and - consider disciplinary mechanisms so that staff are charged for intentional loss of, or damage to equipment and accessories, to make them more accountable for their actions. |
Maintenance, risk-management and safety
Introduction
An effective medical equipment maintenance programme consists of adequate planning, management and implementation. Planning considers the financial, physical and human resources required to adequately implement the maintenance activities. Once the programme has been defined, financial, personnel and operational aspects are continually examined and managed to ensure the programme continues uninterrupted and improves as necessary. Ultimately, proper implementation of the programme is key to ensuring optimal equipment functionality. Box 13 lists the some of the responsibilities and tools typically associated with a medical equipment maintenance system.
Box 13: Summary of registers, ledgers and files used for maintenance and who keeps them
| Equipment users and managers | - Work request/job forms used for recording the details of the request for maintenance support and also used by maintainers to record the work done.
- User department maintenance files to keep track of the requests made and their progress. - User PPM schedules for each type of equipment, PPM timetables and wall calendars. - Equipment cards attached to/kept with all pieces of equipment, showing user PPM and care instructions and service due dates. |
| Maintainers | - Work request/job forms submitted by equipment users and used by the maintainers for recording the details of the work undertaken.
- 'Personal record books[1]' to keep track of special problems and successes with work, which help to identify skill development needs. - PPM schedules for each type of equipment. - Equipment cards attached to/kept with all pieces of equipment used by the maintainers for recording dates when routine inspections, testing, and servicing took place, and are next due. |
| HTM manager (and possibly a clerk) | - Inventories of equipment, furniture, buildings, tools, etc.
- PPM timetables and wall calendars showing when PPM was undertaken on different devices. - Job pending files and job completed files kept by the HTM team to keep track of the requests received and their progress. - A contractors’ record book/file to record which contractor undertook which job, and their performance. - Equipment files and section files which create service histories. - Statistics forms and statistics folders for keeping various statistical records for analysis and for compiling written reports for management. |
| HTM team’s stores-person | - A tools ledger in order to keep a record of: the tools owned, which staff members were allocated which tools, and the return of the tools at the end of the day.
- Stock cards used by the HTM team’s stores-person to record and keep track of the different items stocked in the storeroom. - A stock control ledger kept by the HTM team in order to monitor the quantities of parts and materials used and kept in stock. |
- Simple repairs should be carried out by users. Other repairs should be carried out by clinical engineering personnel. Specialised or complex repairs should be carried out by suppliers.
- Planned preventive maintenance should be carried out to minimise down-time of devices (e.g. to replace x-ray tube at time period recommended by manufacturer rather than when tube fails).
- Calibration and testing should be carried out at regular intervals, as recommended by the manufacturer, by users or clinical engineering personnel – depending on the complexity of the device.
- Records of maintenance and repairs of devices should be documented in a maintenance log, while a plant register is to be maintained for sophisticated or specialised devices.
A maintenance strategy includes procedures for inspection, as well as preventive and corrective maintenance. Performance inspections ensure that equipment is operating correctly, safety inspections ensure the equipment is safe for both patients and operators, and preventive maintenance (PM) aims to extend the life of the equipment and reduce failure rates. Additionally, some hidden problems may be discovered during a scheduled inspection. However, performing inspections of equipment only ensures that the device is in good operating condition at the time of inspection and cannot eliminate the possibility of failure during future use; the nature of most electrical and mechanical components is that they can potentially fail at any time. Corrective maintenance (CM) restores the function of a failed device and allows it to be put back into service.
Risk-based maintenance strategy
A risk-based maintenance strategy prioritises maintenance resources toward assets that carry the most risk if they were to fail. It is a methodology for determining the most economical use of maintenance resources. This is done so that the maintenance effort across a facility is optimised to minimise the total risk of failure.
A risk-based maintenance strategy is based on two main phases:
- Risk assessment.
- Maintenance planning based on the risk.
The maintenance frequency and type are prioritised based on the risk of failure. Assets that have a greater risk and consequence of failure are maintained and monitored more frequently. Assets that carry a lower risk are subjected to a less stringent maintenance programme. By this process, the total risk of failure is minimised across the facility in the most economical way.
Risk is adopted as an index for clarifying priority in risk maintenance[BvR1] technologies. That risk can be given as the product of the probability of failure occurring to the inspected area and the consequence of failure to the surroundings. In other words, risk is the expected value of the degree of impact. Figure 1 shows the concept of distribution of risk held by devices in a system.
Figure 1: Distribution of risk for medical devices
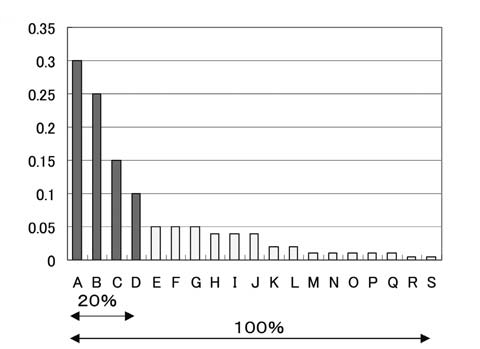
A to S on the horizontal axis are the devices in a system and the vertical axis is the relative risk each device holds. The graph shows that 80% of the total risk of the system is concentrated in just 20% of the devices. In that case, it would be irrational to inspect all devices with the same level of priority. It is thus important to identify the 20% of devices and raise their priority level in the inspection programme. That is the basic concept of risk maintenance technology.
A framework for determining risk in the health industry should be implemented in the following manner:
FIGURE 2: Risk-based maintenance framework
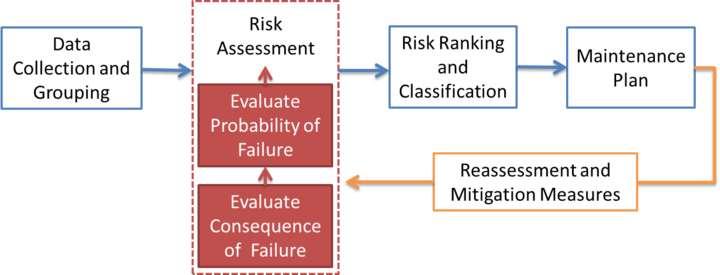
Data collection - equipment groups
For each risk that has been identified data about the risk needs to be collected. In the equipment scenario it is always advisable to group equipment with the same nature and/or function, instead of focusing on individual items. Information per equipment group will include information about the risk, its general consequences and the general methods used to mitigate and predict the risk.
In a model developed as part of the IUSS project the main equipment groups identified are shown in Table 3 (also shown are other factors to be discussed later).
Table 3: Equipment groupings and related risk and maintenance factors
| GRP CODE | GRP DESCRIPTION NDoH | Defined risk | Total risk Factor (%) | % Maint. of repl. value |
| CAUCLE | Auxiliary Cleaning/Sterilising Equipment | B-Medium | 56% | 3.5% |
| CAUOTH | Auxiliary Other Specialised Equipment | B-Medium | 52% | 5.5% |
| CAUSPE | Auxiliary Special Medical furniture | B-Medium | 68% | 3.0% |
| CAUMED | Auxiliary General Medical furniture | C-Low | 20% | 3.0% |
| CAUTES | Auxiliary Test Equipment | B-Medium | 74% | 5.5% |
| CAUFUR | Auxiliary General Administrative Furniture | C-Low | 18% | 2.5% |
| CAUAPP | Auxiliary General Appliances | C-Low | 20% | 3.0% |
| CAUSOF | Auxiliary Software and Communication Systems | B-Medium | 74% | 12.0% |
| CBDAUD | Diagnostic Audiology | B-Medium | 72% | 5.5% |
| CBDFLE | Diagnostic Flexible Endoscopes | B-Medium | 74% | 5.5% |
| CBDMIC | Diagnostic Microscopes | B-Medium | 74% | 5.5% |
| CBDPAT | Diagnostic Pathology Devices | B-Medium | 66% | 5.5% |
| CBDRIG | Diagnostic Rigid Scopes | B-Medium | 62% | 5.5% |
| CBDSCA | Diagnostic Scales | C-Low | 48% | 5.5% |
| CBDSUR | Diagnostic Surgical Lights | B-Medium | 74% | 3.0% |
| CBDVID | Diagnostic Video/Data Devices | B-Medium | 50% | 5.5% |
| CBDOPT | Diagnostic Ophthalmology | B-Medium | 72% | 5.5% |
| CBLANA | Life Support Anaesthesia Units | A-High | 100% | 5.5% |
| CBLBAL | Life Support IABP | A-High | 100% | 5.5% |
| CBLDEF | Life Support Cardioversion Devices | A-High | 88% | 5.5% |
| CBLHRT | Life Support Heart-Lung Bypass Units | A-High | 96% | 5.5% |
| CBLVEN | Life Support Ventilators | A-High | 88% | 5.5% |
| CBMCAP | Monitoring Capnography | B-Medium | 72% | 5.5% |
| CBMCEN | Monitoring Central Stations | B-Medium | 60% | 3.0% |
| CBMECG | Monitoring ECG | B-Medium | 60% | 5.5% |
| CBMMUL | Monitoring Multi-Parameter Monitors | B-Medium | 66% | 5.5% |
| CBMOTH | Monitoring Other Patient Parameters | B-Medium | 64% | 5.5% |
| CBMPRE | Monitoring Pressures | B-Medium | 60% | 5.5% |
| CBMSAT | Monitoring Saturation | B-Medium | 60% | 5.5% |
| CBMTEM | Monitoring Temperature | B-Medium | 60% | 5.5% |
| CBTBEU | Therapeutic Neuromuscular Stimulators | A-High | 76% | 5.5% |
| CBTBRE | Therapeutic Breast Pumps | C-Low | 40% | 5.5% |
| CBTHEM | Therapeutic Hemofiltration Units | A-High | 76% | 5.5% |
| CBTINC | Therapeutic Infant Warming and Phototherapy | A-High | 88% | 5.5% |
| CBTINF | Therapeutic Infusion Devices | B-Medium | 64% | 5.5% |
| CBTINS | Therapeutic Inspired Gas Conditioning Devices | B-Medium | 64% | 5.5% |
| CBTLAZ | Therapeutic Lasers | A-High | 82% | 5.5% |
| CBTWAR | Therapeutic Warmers Patient/Blood/Solution | B-Medium | 74%. | 5.5% |
| CBTDEN | Therapeutic Dental Equipment | B-Medium | 60% | 5.5% |
| CBTPHY | Therapeutic Physiotherapy Equipment | B-Medium | 64% | 5.5% |
| CINELE | Instruments Electric | B-Medium | 74% | 5.5% |
| CINMAN | Instruments Manual | B-Medium | 62% | 2.0% |
| CINPHN | Instruments Pneumatic | B-Medium | 74% | 5.5% |
| CINHOL | Instruments Hollow ware & General Stainless ware | C-Low | 20% | 2.0% |
| CRDAUX | Radiology Digital Auxiliary Equipment | B-Medium | 72% | 9.0% |
| CRDBDE | Radiology Digital Bone Densitometers | A-High | 80% | 5.5% |
| CRDCAT | Radiology Digital Computed Tomography | A-High | 84% | 9.0% |
| CRDDEN | Radiology Digital Radiographic Units Dental | A-High | 80% | 9.0% |
| CRDMRI | Radiology Digital Magnetic Resonance Imaging | A-High | 84% | 9.0% |
| CRDRFS | Radiology Digital Radiographic/Fluoroscopic Systems | A-High | 84% | 9.0% |
| CRDRSD | Radiology Digital Radiographic Systems | A-High | 80% | 9.0% |
| CRDULT | Radiology Digital Ultrasonic Scanning Systems | A-High | 76% | 6.0% |
| CRDNUC | Radiology Nuclear Medicine Equipment | A-High | 84% | 9.0% |
Risk evaluation - factors impacting on risk
At the risk-evaluation stage, both the probability of the risk and the consequence of the risk are quantified in the context of the equipment group under consideration. For this specific model, more emphasis has been placed on risk factors impacting directly on the maintenance function.
Volume
Availability of alternative devices to perform the same required function
Score Intensity
5 - Very low volume (only one per hospital).
4 - Low volume.
3 - Medium (loan units available).
2 - Moderate (some duplication exist).
1 - High volume (high numbers per unit).
Function
Equipment function has a direct impact on the risk associated with the equipment:
Score Function
Therapeutic equipment
5 – Life-support.
4.5 - Surgical and intensive care.
4 - Physical therapy and treatment.
Diagnostic equipment
3.5 - Surgical and intensive care monitoring. 3 - Additional physiological monitoring and diagnostic.
Analytical equipment
2.5 - Analytical laboratory.
2 - Laboratory accessories.
1.5 - Computer and related.
Miscellaneous equipment
1 - Patient Related Furniture and Other.
Physical risk
The risk associated with equipment failure and/or misuse measured by the impact on patient and/or staff injury
Score Risk
5 - Patient death.
4 - Patient or operator injury.
3 - Inappropriate therapy or misdiagnosis.
2 - Operational risk.
1 - No significant risk.
Maintenance intensity
Maintenance intensity in the operational environment also impacts on the risk model due to the specific requirements placed on scarce resources such as technical staff and financial constraints.
Score Intensity
5 - Extensive and costly with highly skilled technical staff involved.
4 - Moderate skilled technical resources required but still costly.
3 - Average skilled technical resources required with low cost associated.
2 - Low technical skill required with low cost.
1 - Minimal maintenance required.
Technology intensity
This is the risk associated with the requirements placed on the operator due to the nature and technology level of the equipment. Qualified operators and trained staff remain a scarce resource in the medical device industry.
Score Intensity
5 - Operator-certified and specialised training required.
4 - Specialist nursing training required.
3 - Average training required.
2 - Very low skill level required.
1 - No training required.
Risk ranking and risk classes
With the risk evaluation complete, the probability and consequence are combined to determine the total risk. This total risk is ranked against pre-determined levels of risk. As a result, the risk is either acceptable or unacceptable.
Equipment groups can therefore be divided into three major classes according to the risk evaluation outcome:
High-risk equipment – risk score of 75% and higher
- Risk is not acceptable and preventive maintenance and condition monitoring is highly advised.
- This category includes all life-support devices, key resuscitation devices, and other devices whose failure or misuse is reasonably likely to result in serious injury to patients or staff.
- High impact on hospital operational activities.
- Low volume of items increases risk.
- Device history could increase or reduce risk.
Medium-risk equipment – risk score of 50% to 75%
- Risk is not acceptable and preventive maintenance and/or condition monitoring advised.
- This category includes devices whose misuse, failure, or absence would have significant impact on patient care, but would not likely cause immediate serious injury.
- Medium impact on hospital operational activities.
- Low volume of devices raises risk.
- Device history could increase or reduce risk.
Low-risk equipment – risk score of 50% and lower
- Risk is acceptable and very low level of preventive maintenance – if any maintenance is advised.
- This category includes devices whose failure or misuse is unlikely to have serious consequences.
- Very low impact on hospital operational activities.
- High volume of devices reduces risk.
Inspection and preventive maintenance planning
Maintenance planning should always start with a basic approach were where only staff and maintenance material are required, and further evolve to a more advanced approach where the specific asset and method required for the asset are addressed. This also forms the basis for the maintenance excellence hierarchy. The preventative maintenance planning is based on the following diagram:
FIGURE 3: The maintenance planning process
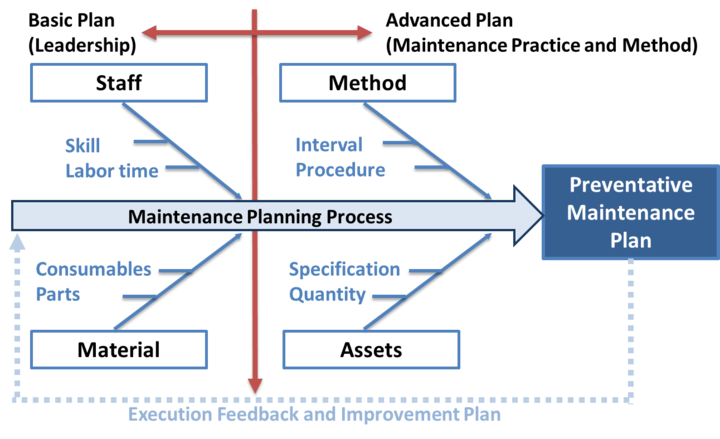
Equipment supported
The starting point for any advanced maintenance approach is to know exactly what is being maintained. The model therefore makes use of Standard Equipment List(s) obtained from NDoH. The Standard Equipment List (SEL) per level of care is a list of the medical equipment in the various nursing departments and provides detail per room level. The SELs for the various levels of care are combined in the model and standardised according to Universal Medical Device Nomenclature System™ (UMDNS). The combined equipment list is grouped according to the various equipment groups and the risk model applied.
Final quantities of equipment determined by number of facilities to achieve a theoretical equipment list per province/district. This theoretical HT Asset Register can now be further manipulated to accept changes made to quantities of specific unique items.
Method - maintenance procedures and intervals
Maintenance procedures and intervals are determined by the manufacturer and/or legal requirements. There are international minimum accepted norms available to determine not only the service/inspection interval (Table 4) but also the minimum procedure to be performed (NB with Table 4: All other types: once a year).
Table 4: Maintenance intervals for different equipment types
| Equipment type | Interval p.a. | Equipment type | Interval p.a. |
| Anaesthesia Unit Vaporisers | 2 | Infusion Devices | 1 |
| Anaesthesia Unit Ventilators | 2 | Intra-Aortic Balloon Pumps | 2 |
| Anaesthesia Units | 2 | Isolated Power Systems | 2 |
| Apnoea Monitors | 1 | Laparoscopic Insufflators | 1 |
| Argon-Enhanced Coagulation Units | 1 | Mammography Units | 2 |
| Argon Surgical Lasers | 2 | Medical Gas/Vacuum Systems | 1 |
| Aspirators, Emergency and Tracheal | 2 | Mini C-arms | 2 |
| Automated External Defibrillators | 2 | Mobile C-arms | 2 |
| Autotransfusion Units | 2 | Mobile High-Efficiency-Filter Air Cleaners | 4 |
| Beds, Electric | 1 | Mobile x-ray Units | 2 |
| Blood Pressure Monitors, Electronic Indirect | 1 | Nd:YAG Surgical Lasers | 2 |
| Blood Pressure Monitors, Invasive | 1 | Oxygen-Air Proportioners | 1 |
| Blood/Solution Warmers | 1 | Oxygen Analysers | 1 |
| Capnometers and Multiple Medical Gas Monitors | 1 | Oxygen Concentrators | 4 |
| Carbon Dioxide Surgical Lasers | 2 | Pacemakers, External Invasive | 1 |
| Cardiac Resuscitators | 2 | Pacemakers, External Non-invasive | 2 |
| Centrifuges | 1 | Peritoneal Dialysis Units | 2 |
| Circulating-Fluid Pumps | 1 | Phototherapy Units | 1 |
| Circumcision Clamps | 1 | Physical Therapy Ultrasound Units | 1 |
| Conductive Furniture and Floors | 12 | Pneumatic Tourniquets | 2 |
| Critical Care Ventilators | 2 | Portable Ventilators | 2 |
| Cryosurgical Units | 1 | Pressure Transducers | 2 |
| Defibrillators | 2 | Pulmonary Resuscitators, Gas-Powered | 1 |
| ECG Monitors | 1 | Pulmonary Resuscitators, Manual | 1 |
| Electrical Receptacles | 1 | Pulse Oximeters | 1 |
| Electrocardiographs | 1 | Radiant Warmers | 1 |
| Electrosurgical Units | 2 | Radiographic Units, General Purpose | 2 |
| Fetal Monitors | 1 | Radiographic/Fluoroscopic Units, General-Purpose | 2 |
| Frequency-Doubled Nd:YAG Surgical Lasers | 2 | Smoke Evacuators | 1 |
| Heart-Lung Bypass Units | ³4 | Sphygmomanometers | 1 |
| Heated Humidifiers | 1 | Suction Regulators, Tracheal* | 2 |
| Hemodialysis Units | 4 | Temperature Monitors | 1 |
| Ho:YAG Surgical Lasers | 2 | Traction Units | 1 |
| Hypo/Hyperthermia Units | 1 | Transcutaneous O2/CO2 Monitors | 1 |
| Infant Incubators | 1 | Ultrasound Scanners | 1 |
Materials – service required items
Material required for preventive maintenance is very device- and manufacturer-specific and cannot be generalised or averaged to be used in the model. The majority of the maintenance and repair workload is simple tasks requiring common spare parts. Therefore, try to remember that more than 80% of all spare parts needed (in theory), represent less than 20% of the overall spare parts cost. In other words, only 20% of your maintenance and repair tasks can eat up 80% of your spare parts budget, because the equipment is complex and expensive.
Staff – human resource requirement
The maintenance and repair requirements of healthcare facilities are more demanding than those of most other facilities. The high intensity of use, long operating hours (24-hours per day for most primary care hospitals) and high-technology specialised equipment all lead to a relatively high maintenance workload usually fulfilled by a combination of internal staff and external service providers.
There are a number of statistical models to determine the ideal staff level but most fail owing to the complexity of the different variables:
- Technology level of hospital.
- Availability of internal maintenance specialisation.
- Internal vs outsourced maintenance combination.
- Cost constraints.
- Etc.
To get a better estimation of staff levels as well as the impact of outsourced maintenance, this model takes a number of specific factors related to staff into consideration:
Maintenance intensity of specific equipment
The combined theoretical equipment list contains a total of 768 unique equipment descriptions. Each equipment type has been graded according to the service requirement assigned by the service procedure and interval, as follows:
Table 5: Grading of equipment service requirements
| Level | Major Service | Minor Service | Verification | Predictive Inspection | Reactive Maintenance |
| 1 | XXX | XXX | XXX | XXX | XXX |
| 2 | XXX | XXX | XXX | XXX | |
| 3 | XXX | XXX | XXX | ||
| 4 | XXX | XXX | |||
| 5 | XXX |
- Major service
A service that normally requires the replacement of parts as well as consumable service items.
- Minor service
A service that normally only requires the replacement of consumable service items.
- Verification
The verification of equipment performance to a standard in the form of a performance analyser and/or simulator.
- Predictive inspection
The inspection of equipment to a set list. No tools and/or verification equipment required.
- Reactive maintenance
Only reactive work performed on equipment breakdown and/or failure.
The specific level of maintenance is always determined by the highest service requirement and may not always include the lower service requirements. A device that requires a major service but no minor services or verification will therefore be graded as Level 1 maintenance intensity, although not all services are required.
Maintenance trade classification
The specific staff skill required as well as the skill level is assigned to the service requirement of every unique equipment description.
- Electronic high
Electronically qualified person with specialisation on specific equipment.
- Electronic medium
Electronically qualified person without specialisation on specific equipment.
- Electronic low
Person with electronic background but no formal tertiary qualification.
- Mechanical high
Mechanically qualified person with specialisation on specific equipment.
- Mechanical medium
Mechanically qualified person without specialisation on specific equipment.
- Mechanical low
Person with mechanical background but no formal tertiary qualification.
Maintenance labour time requirement
An average of labour time per unique equipment description is calculated based on equipment group risk (maintenance intensity) and assigns the following values per service type:
Table 6: Labour hours per service type
| Labour hours | Maintenance intensity risk score | ||||
| Service type | 1 | 2 | 3 | 4 | 5 |
| Major service | 1 | 1.5 | 2.5 | 3.5 | 4.5 |
| Minor service | 0.5 | 0.75 | 1.25 | 1.75 | 2.25 |
| Verification | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| Predictive maintenance | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 |
The only equipment group that does not form part of the above table is radiology equipment, where equipment verification has been extended to an average of two hours per verification.
Outsourcing of maintenance activities
Many equipment types require very specialised training and tools for preventative as well as reactive maintenance. This high specialisation requirement on the maintenance often leads to the outsourcing of maintenance. Outsourcing could occur per equipment group or per unique device (manufacturer specific).
This model incorporates three different categories that can be user selected per unique equipment type:
- Outsourced
All maintenance activities related to the specific equipment are performed by outsourced service providers.
- Combined
The major and minor services as well as reactive maintenance are performed by outsourced service providers and verification and predictive maintenance are performed by internal staff. As a norm, the ratio of outsourced labour to internal labour is set at 70% to 30%. The ratio can be changed to accommodate various scenarios.
- Internal
All maintenance activities performed by internal staff with the exception of some reactive maintenance outsourced (unique cases).
The various types of outsourcing contracts include:
- Full coverage contracts shall be considered contracts which include both unscheduled (emergency repair) and scheduled (preventive/interval) maintenance. This type of coverage will involve the least amount of work for the operator and the Health Technology/Clinical Engineering Department, as it will be up to the vendor to complete all maintenance of the equipment. The time invested in the management of the contract will involve the most effort by the Health Technology Department as the billing will have to be monitored and the contract will need to be overseen to ensure that all provisions of the contract(s) are being carried out in a timely manner.
- Unscheduled maintenance only (emergency repair) contracts are desirable where the ability to perform the scheduled maintenance programme exists within the hospital. Each item of equipment should receive a minimum of one (1) preventive maintenance inspection per year by the internal staff.
- Scheduled maintenance only (preventive/interval maintenance inspection) contracts shall be considered cost-effective where the need for expensive specialised test equipment is needed and is not owned by the hospital, and/or where the reliability of the equipment is such that the unscheduled downtime of the equipment does not warrant the need for full coverage. Cost-effectiveness shall be a major part in this decision.
- Parts only contracts shall be considered where the expertise to perform the work required is contained within the facility but the cost of parts is expensive. The decision to acquire this type of contract shall be supported by documentation to the cost of parts and the capability of the Biomedical Engineering staff.
- Variations of and combinations of the aforementioned are another possibility. Where documentation is present, it is possible to decide the most cost-effective method of coverage for each piece of equipment. The extent of coverage and any portion of the workload which can be performed by the in-house staff shall be prime consideration to the exact type of coverage required.
Implementation of preventative maintenance plan
There are various combinations of outsourced maintenance and the planner needs to be very aware of the different impact these combinations might have on internal staffing levels. A phased approach according to equipment risk level should form the baseline for implementation.
It would be impractical and not cost-effective to include all equipment in a preventive maintenance plan; if the risk is acceptable then the specific equipment should not form part of an IPM plan and only reactive maintenance should be performed. We can divide the equipment list into groups for specific action, as shown below (sub-types have been removed, e.g. Radiographic Units, Dental, Extraoral and Radiographic Units, Dental, Intraoral are covered by the term Radiographic Units, Dental[BvR1] ) for the sake of brevity; the full lists are available on request. For each equipment type, there are established procedures and protocols for what needs to be done – these are available from organisations such as ECRI.
A. High-risk with high maintenance intensity – typical outsourcing with comprehensive service contracts and short-term implementation:
o Anesthesia Units
o Angioplasty Systems
o Apheresis Units
o Autotransfusion Units
o Brachytherapy Systems
o Circulatory Assist Units, Cardiac, Intra-Aortic Balloon
o Collimators, Radiographic
o Densitometers, Bone[BvR2]
o Detectors, Fluid Level, Heart-Lung Bypass Unit
o Heart-Lung Bypass Units
o Heat Exchangers, Heart-Lung Bypass
o Hemodialysis Units
o Peritoneal Dialysis Units
o Positive Airway Pressure Units, Continuous
o Pulsatile Pressure Generators, Heart-Lung Bypass
o Radiographic Systems/Units
o Radiographic/Fluoroscopic Systems/Units
o Radiotherapy Beam Block Shaping Systems
o Radiotherapy Simulation Systems
o Radiotherapy Systems, Linear Accelerator
o Scanning Systems, Computed Tomography
o Scanning Systems, Gamma Camera
o Scanning Systems, Magnetic Resonance Imaging
o Stereotactic Systems
o Ventilators.
B. High-risk with medium maintenance intensity – typical outsourcing of major services, verification and inspections performed by internal staff and short-term implementation:
o Defibrillators
o Echocardiographs
o Incubators
o Inflators, Angioplasty Balloon
o Lasers
o Pacemakers, Cardiac, External, Invasive Electrodes
o Phototherapy Units
o Programmer/Testers, Implantable Cardiac Pacemaker
o Scanning Systems, Ultrasonic
o Phantoms, Computed Tomography
o Nerve Locators
o Stimulators, Electrical
o Valves, Positive End-Expiratory Pressure.
C. High, high risk with low maintenance intensity – typical reactive maintenance performed by internal staff with parts-only contracts as possibility and medium- to long-term implementation:
o Angioscopes
o Brachytherapy Applicators/Sources
o Extractors, Plasma
o Masks, Resuscitator
o Radioisotope Transfer Units
o Resuscitators, Pulmonary, Manual, Reusable
o Transducers, Ultrasonic.
D. Medium-risk with high maintenance intensity - typical outsourcing of major services, verification and inspections performed by internal staff and medium- to long-term implementation:
o Aerators, Ethylene Oxide
o Analysers, Laboratory
o Cabinets, Biological Safety
o Cameras, Identification
o Chromatography Systems
o Cleaners, Bedpan
o Compressors
o Computer Aided Detection Systems, Papanicolaou Smear
o Detectors, Air Bubble/Foam, Heart-Lung Bypass Unit
o Disinfecting Units, Liquid, Flexible Endoscope
o Injectors, Contrast Media
o Laminar Air Flow Units
o Lithotripters
o Microscopes, Light, Operating
o Microwave Therapy Systems, Tissue Ablation, Prostatic
o Pill Counters, Automatic
o Printers, Dry-Processing
o Radiofrequency Therapy Systems
o Sterilising Units
o Stools, Adjustable, Dentistry
o Tables, Imaging, Ultrasound
o Tables, Operating
o Telemanipulation Systems, Surgical
o Thrombometers
o Tissue Processors
o Urodynamic Measurement Systems.
E. Medium-risk with medium/low maintenance intensity - typical maintenance performed by internal staff and long-term implementation.
F. All low-risk equipment – reactive maintenance only.
Computerised maintenance management system (CMMS)
In most modern health-care facilities, the number of pieces of medical equipment and the number of service events are so large that keeping and organising this information can only be done by a computer system. Thus, a computerised maintenance management system (CMMS) can be very useful in managing the medical equipment maintenance programme.
A CMMS is made up of fields, tables and modules populated with data from the clinical engineering or medical equipment department of a given facility. Using a CMMS, critical data can be accessed, manipulated and analysed using user-friendly interfaces. Reports can be generated from the system to help policy-makers reach decisions regarding health technologies. However, it is important to take into consideration multiple factors when deciding to adopt and develop a CMMS. Factors such as financial and technical resources are important when determining whether to purchase a commercial product, use open-source software, or to develop a system locally. Implementation requires proceeding through a number of phases that will allow the system to be planned comprehensively. By completing this multistep process, the options for deployment will be thoroughly evaluated; a suitable package will be selected, installed and customised; data will be entered; and training on the CMMS will be provided.
For organisations with the appropriate resources to implement this tool, a CMMS can be very beneficial. It is a highly flexible tool that when properly implemented has the ability to transform the management of medical equipment while also improving the availability and functionality of the technology required to prevent, diagnose and treat illness.
A CMMS can be used to:
- standardise and harmonise information within a HTM programme;
- assist in the planning and monitoring of inspection and preventive maintenance, and schedule and track repairs;
- monitor equipment performance indicators such as mean time between failures, downtime and maintenance costs for individual or equipment groups of the same model, type or manufacturer;
- monitor clinical engineering staff performance indicators such as repeated repairs by the same staff member for the same problem, average downtime associated with individuals, and productive work time for individuals or groups;
- generate reports that can be used to plan user training programmes based on equipment failure trends in certain departments or health facilities;
- host libraries of regulatory requirements and safety information;
- generate the appropriate documentation for accreditation by regulatory and standard organisations; and
- generate reports to assist in the monitoring and improvement of the productivity, effectiveness and performance of HTM
CMMS fields and tables
A field is a single piece of information, for example an “equipment serial number”. A table is a collection of related fields; for example, an equipment location table might be made up of the fields “building”, “department” and “room” where a piece of equipment is stored.
To avoid long descriptive text, it is useful to develop a comprehensive, consistent and simple coding system for the various activities of the database. A single code is a field, and a collection of fields can be organised into tables. Coding of tables can be developed for equipment inventory, personnel, maintenance procedures and equipment locations.
Commercial CMMS packages normally have a set of generic codes that can be adapted or customised according to the needs of the health facility. For “equipment type” coding, standard nomenclature such as the Universal Medical Device Nomenclature System and the Global Medical Device Nomenclature System should be considered. Implementing appropriate nomenclature can also facilitate the management of alerts and vigilance reports. A generic list of fields that are commonly included in a CMMS is provided in Table 7.
Table 7: List of generic fields for a CMMS
| Item | Fields |
| Equipment type | Equipment type |
| Inspection and preventive | |
| maintenance (IPM) procedures | |
| IPM frequency | |
| Risk level | |
| Responsible staff | |
| Equipment model | Model number |
| Serial number | |
| Parts list | |
| Parts code and name | |
| IPM procedures | |
| Manufacturer | Manufacturer code and name |
| Supplier code and name | |
| Manufacturer email, telephone and address | |
| Supplier email, telephone and address | |
| Manufacturer contact name | |
| Supplier contact name | |
| Stores/spares | Store code and name |
| Parts code and name | |
| Parts order number | |
| Staff | Employee code |
| Employee name | |
| Employee position | |
| Access level | |
| Training details | |
| Maintenance | Inventory number |
| Work order number | |
| Service provider | |
| Service engineer code | |
| Training details | |
| Service | IPM procedures |
| IPM interval | |
| Service provider | |
| Training details | |
| Health facility | Facility code and name |
| Area | |
| Department |
CMMS modules
A module is a collection of tables and data screens. The inventory module, for example, is made up of the “equipment type” table, the “manufacturer information” table and the “equipment location” table. The following lists the basic modules of a CMMS package:
i. Equipment inventory module.
ii. Spare parts inventory and management module.
iii. Maintenance (repair) module.
iv. Contract management module.
v. Preventive maintenance module.
vi. Reporting module.
CMMS implementation
Clinical engineering staff must be included in the entire CMMS planning and implementation process, as shown in Figure 4 below and described thereafter.
Figure 4: CMMS planning & implementation process
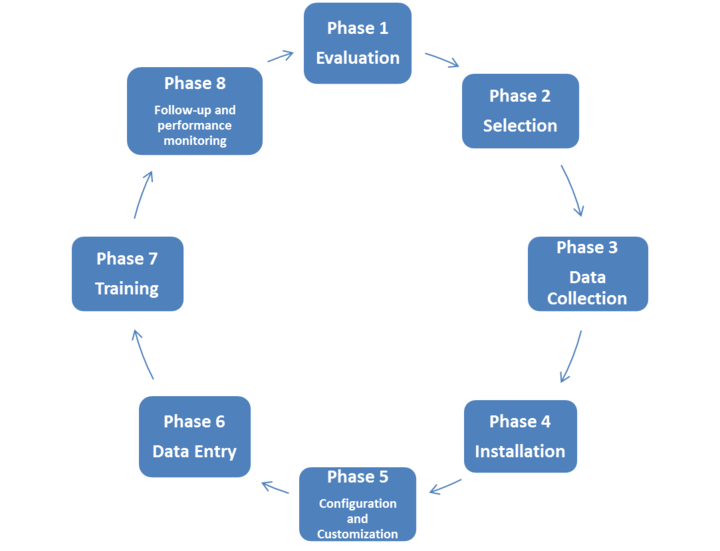
Phase 1 - Evaluation
It is important to conduct a feasibility study to evaluate and assess the need for a CMMS. During this phase, a complete analysis is conducted and the scope of the system is defined.
Phase 2 - Selection
Once specifications for a system have been identified, an appropriate package can be selected. It may be one that is commercially available, customised to the health facility’s needs or designed specifically for the user.
Phase 3 – Data collection
A comprehensive survey and analysis of all available data should be conducted before implementing the CMMS. Complete and current inventory lists as well as all other tables required by the CMMS must be available to avoid costly data collection processes. Most CMMS solutions fail due to inaccurate and incomplete data collection.
Phase 4 – Installation
The CMMS can be implemented as a complete system, by individual modules, by equipment type or by location. This is the decision of the clinical engineering department and will depend on the resources available and hardware configuration.
Phase 5 – Configuration and customisation
Configuration of the system could cover areas such as simple workflow, access and security, and user preferences. Customisation refers to the technical functional requirements of the system including custom screens and tables, facility-specific workflow and additional data fields.
Phase 6 – Data entry
Initial data entry of comprises common fields such as equipment model number, inventory code, human resources, equipment locations, manufacturer information and nomenclature classifications. User security levels and associated passwords, access levels and access types are also set at this stage.
Phase 7 – Training
Basic and advanced functional training can now be performed. It is important that each staff member of the clinical engineering department is fully confident and familiar with all functions of the CMMS and specific level of functionality assigned to him/her. It is useful to begin staff training in the early stages of implementation to increase staff buy-in and improve confidence.
Phase 8 – Follow up and performance monitoring
Continuous monitoring of the system is conducted to ensure that it is directly contributing to the improvement and effective running of the HTM programme.
Human resources
To develop an appropriate and complete staff establishment in order to achieve the objectives of the maintenance strategy is challenging and an ongoing optimisation process – even more so under a future NHI dispensation. The model presented here can be used to determine staff requirements and plan for future development; however, actual implementation will highly depend on the following factors:
- Equipment included in preventative maintenance plan.
- Mixture of in- and outsourced maintenance.
- Availability of operational technically qualified staff.
- Availability of management and support staff.
- Specific health technology department structure; centralised vs decentralised structures.
- Funds available to acquire resources.
The provincial reviews have indicated that most provinces follow a combination approach between Centralised and Decentralised Health Technology Department structures, idealised in Figure 5 below.
The Central Maintenance Unit(s) provide a single contact point to Health Facilities while decentralised HT Departments service across multiple Health Facility Locations. The Tertiary Hospital in most cases being the exception to the rule where the HT Department services the Tertiary Hospital only and form a self-supporting unit.
The Central Maintenance Units are responsible for management responsibilities which includes client relationship management, regulatory compliance, uniform standards of delivery and management of human and financial resources.
Figure 5: Idealised health technology department structure
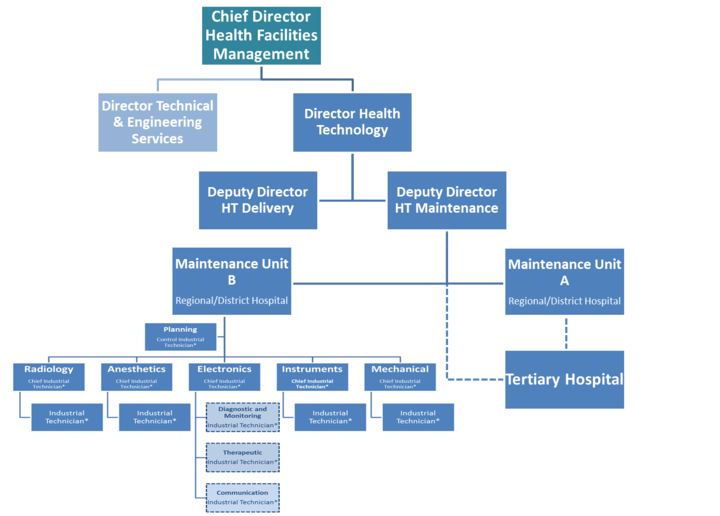
Self-supporting (stand-alone) decentralised departments - tertiary hospitals
Properties:
- Tertiary hospitals normally have intra-hospital HT departments without provincial support.
- Flat management structure – not included in staff complement above.
- Manage own service contracts and spare parts.
- High level of specialisation.
A suggested staff complement is shown in Table 8 (this assumes a typical asset inventory and allows for two-thirds of available time spent on maintenance), and a suggested organisational structure is shown in Figure 6 below.
Table 8: Typical staff complements (technical) for a self-supporting HT maintenance department
| Equipment risk level | ||||
| Resource | C-Low | B-Medium | A-High | Combined |
| Electronic high | 0.00 | 3.82 | 5.68 | 9.50 |
| Electronic medium | 0.44 | 4.28 | 0.81 | 5.53 |
| Electronic low | 0.03 | 0.02 | 0.00 | 0.04 |
| Mechanical high | 0.00 | 1.14 | 0.01 | 1.15 |
| Mechanical medium | 1.09 | 3.12 | 0.01 | 4.22 |
| Mechanical low | 0.28 | 0.06 | 0.00 | 0.34 |
| Total | 20.79 | |||
Figure 6: Typical organisational structure for a self-supporting HT maintenance department
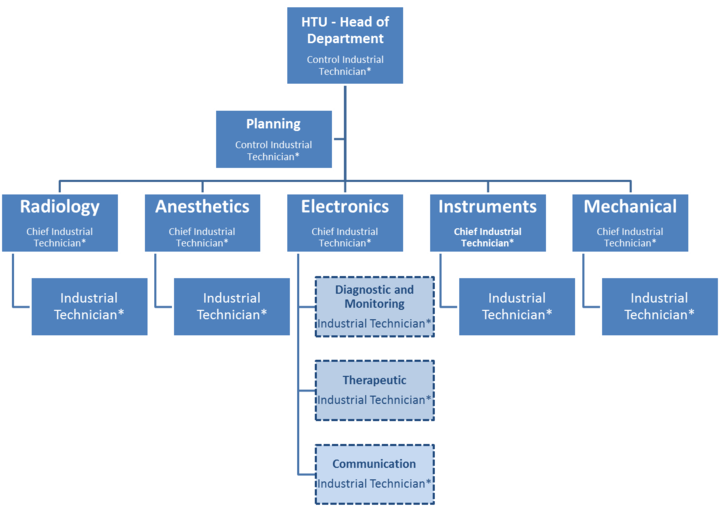
Centralised maintenance units
There are a number of variations of the Central Maintenance Units, all depending on the specific size of the area serviced:
- Provincial HT maintenance units.
Servicing all levels of facilities across various districts.
- District HT maintenance units.
Servicing all levels of facilities within a specific district.
- Facility HT maintenance units.
Servicing a single facility where it’s based and a couple of smaller facilities in the direct surrounding area.
The typical staffing complement will depend on the type and quantity of the facilities supported by the central HT maintenance unit. Typical staffing levels per facility based on the variables in the model are suggested in Tables 9-12 below, and the typical structure of a central HT maintenance unit is shown in Figure 7.
Table 9: HT maintenance staffing requirement (regional hospital)
| Equipment risk level | ||||
| Resource | C-Low | B-Medium | A-High | Combined |
| Electronic High | 0.00 | 0.42 | 0.78 | 1.20 |
| Electronic Medium | 0.08 | 1.01 | 0.18 | 1.27 |
| Electronic Low | 0.00 | 0.01 | 0.00 | 0.01 |
| Mechanical High | 0.00 | 0.32 | 0.00 | 0.32 |
| Mechanical Medium | 0.34 | 0.26 | 0.00 | 0.60 |
| Mechanical Low | 0.06 | 0.01 | 0.00 | 0.08 |
| Total | 3.47 | |||
Table 10: HT maintenance staffing requirements (district hospital)
| Equipment Risk Level | ||||
| Resource | C-Low | B-Medium | A-High | Combined |
| Electronic High | 0.00 | 0.32 | 0.26 | 0.59 |
| Electronic Medium | 0.05 | 0.49 | 0.15 | 0.70 |
| Electronic Low | 0.00 | 0.00 | 0.00 | 0.01 |
| Mechanical High | 0.00 | 0.15 | 0.00 | 0.16 |
| Mechanical Medium | 0.16 | 0.60 | 0.00 | 0.76 |
| Mechanical Low | 0.03 | 0.01 | 0.00 | 0.04 |
| Total | 2.25 | |||
Table 11: HT maintenance staffing requirements (community health centre)
| Equipment Risk Level | ||||
| Resource | C-Low | B-Medium | A-High | Combined |
| Electronic High | 0.00 | 0.05 | 0.20 | 0.25 |
| Electronic Medium | 0.05 | 0.13 | 0.08 | 0.25 |
| Electronic Low | 0.00 | 0.00 | 0.00 | 0.01 |
| Mechanical High | 0.00 | 0.03 | 0.00 | 0.03 |
| Mechanical Medium | 0.16 | 0.08 | 0.00 | 0.24 |
| Mechanical Low | 0.10 | 0.00 | 0.00 | 0.11 |
| Total | 0.88 | |||
Table 12: HT maintenance staffing requirements (large clinic, with maternity)
| Equipment Risk Level | ||||
| Resource | C-Low | B-Medium | A-High | Combined |
| Electronic High | 0.00 | 0.03 | 0.02 | 0.05 |
| Electronic Medium | 0.01 | 0.05 | 0.01 | 0.07 |
| Electronic Low | 0.00 | 0.00 | 0.00 | 0.00 |
| Mechanical High | 0.00 | 0.04 | 0.00 | 0.04 |
| Mechanical Medium | 0.02 | 0.03 | 0.00 | 0.05 |
| Mechanical Low | 0.02 | 0.00 | 0.00 | 0.02 |
| Total | 0.23 | |||
Figure 7: Typical structure for a central HT maintenance unit
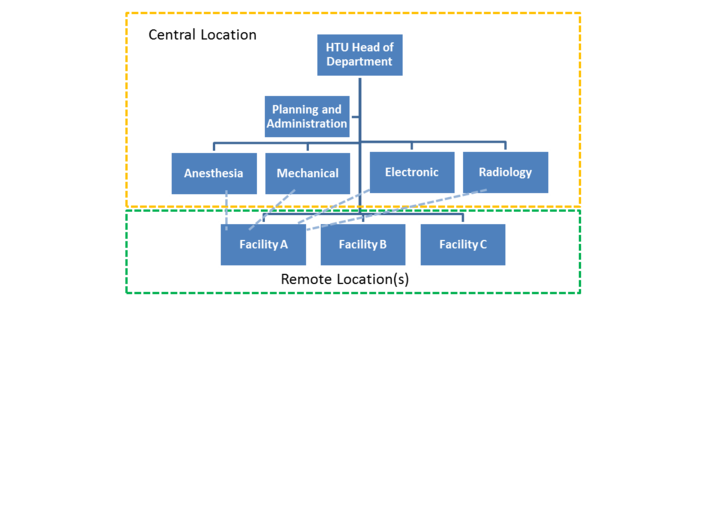
Properties:
- Size depends on number of facilities.
- Specialisation not as advanced as in tertiary structure.
- Staff at central location and/or remote locations.
- Specific functions at central to support remote staff.
- Resources at remote locations can be increased with staff from central location to relieve short period demand(s).
- Administration and planning of all activities from central location.
Replacement, decommissioning and disposal
Introduction
- Planning for replacement of a device should commence almost at the time of the original purchase to allow adequate time for budgeting and to prevent interruptions or disruption of services. Planned replacement can be based on the projected life-span of the device locally.
- Decommissioning should be carried out systematically with set procedures and processes of certification, also ensuring that services are not interrupted.
- Disposal of devices should be carried out systematically with set procedures and processes, with safety being a prime concern.
- Additional precautions must be taken for disposal of special devices, e.g. radioactive devices.
Replacement of equipment
Replacement is necessary because all equipment has a finite life expectancy. This lifespan will depend upon the type of equipment, and the types of technology contained within it. For example, five years might be the typical life for an ECG monitor, 10 years for a suction pump, 15 years for an operating table, and 20 years for an electricity generator. Once the equipment reaches the end of its life no amount of intervention (such as maintenance) will be effective, and the only option will be to replace it. International guidance on equipment lifetimes is available, but experience has shown that lifetimes for individual items are crucially dependent on their utilisation, care and maintenance.
If replacement of equipment is not planned for, the health service delivered to the public may deteriorate. If equipment is not replaced at the end of its life, there will be:
- an uneven standard of reliability among the equipment in a facility or service, and
- a general deterioration in:
- performance;
- safety;
- dependability; and
- availability for use.
For all medical devices and equipment, a stage is reached at which replacement must be considered. If any of the following seven criteria apply, the medical device/equipment is no longer serviceable:
- Worn out beyond economic repair.
- Damaged beyond economic repair.
- Unreliable (check service history).
- Clinically or technically obsolete.
- Spare parts no longer available.
- More cost-effective or clinically effective medical devices/equipment have become available.
- Unable to be cleaned effectively prior to disinfection and/or sterilisation.
If the medical device/equipment survives this test, a date should be set for re-testing (e.g. in a year's time).
Each facility should replace equipment for valid reasons only, which should be defined. Box 14 lists some such reasons and suggests criteria for condemning and replacing equipment. Equipment must NOT be condemned and/or disposed of on the strength of a Report or Certificate issued by the manufacturer, agent, supplier or contractor.
Box 14: Example of valid reasons for condemning and replacing equipment
| Valid replacement criteria
i. Equipment will be replaced only when one of the following valid reasons has been fulfilled: a. it is worn out beyond repair (has reached the end of its natural life); b. it is damaged beyond repair; c. it is unreliable – faulty, old, unsafe; d. it is clinically or technically obsolete; e. spare parts are no longer available; and f. it is no longer economical to repair. and one of the following valid reasons has also been fulfilled: g. utilisation statistics are available to show that it is still required; and h. a demonstrated clinical or operational need still exists.
Judging when it is time to condemn equipment Senior maintenance staff need to study the equipment, and judge:
|
Decommissioning and disposal of equipment
Formal procedures must exist for condemning and disposal of equipment. Failure to dispose of equipment properly could result in the following:
- graveyards of abandoned equipment piling up around health facilities;
- departments, store rooms, cupboards, and workshops full of old equipment; and
- previously condemned equipment ending up back on the wards and being re-used.
Once equipment has been condemned, a formal policy is needed to oversee its disposal. This should cover:
- how it should be disposed of safely;
- how it can be disposed of as promptly as possible;
- how it can be disposed of in an environmentally sound way according to a “waste management and hygiene plan”; and
- how useful spare parts can be stripped off before the equipment is disposed of.
Note: The practice of selling intact condemned medical equipment to contractors is strongly discouraged. Where possible and appropriate, the condemned medical equipment must be destroyed and sold as scrap to a metal dealer.
Flowchart 11 below describes the typical decommissioning process.
The condemning and disposal of equipment should trigger the purchase of a replacement piece of equipment. It is preferable to plan for replacements before they are needed and, where possible, likely replacement needs should be identified within the annual equipment inventory update and annual plans. These activities should be timed to take place ahead of the next procurement cycle, which usually takes place annually.
In summary, to replace and dispose of equipment it is necessary to have the following:
technical skills to identify those items ready for replacement;
- good procurement practices which enable financing and purchase of replacement items in good time;
- courage and determination to take equipment out of service when necessary, even if the users want to keep using it;
- a formal method for condemning equipment;
- a formal method for disposing of the equipment, safely and in an environmentally sound way; and
- a formal method so that the disposal of equipment triggers the purchase of a replacement item.
Flowchart 11: Steps in a typical decommissioning process

Monitoring & evaluation of the HTM system
This document has outlined the building blocks of an effective, evidence-based and responsive Health Technology Management (HTM) system. This system – in its pivotal contributing role to safe, cost-effective, accessible and quality healthcare service delivery - needs to be monitored and evaluated on an ongoing basis at all levels of care delivery.
Annex III suggests a set of Standards that align with the sections in this document; they also underline the need for a clear statement of vision and intended performance. They also indirectly point to the need for adequate and proper resourcing – at all levels of delivery – of the structures, processes, activities and human capital that together constitute an HTM system.
Annex III also contains a list of HT-related Indicators that could be used to measure/assess compliance with these Standards as well as indicate levels of performance in their own right.
These HT-related Standards and Indicators are particularly relevant with respect to the newly established Office of Standards Compliance of the Department of Health and the associated National Core Standards for Health Facilities. They will also be very useful as bridging links between the public and the private sectors as we move towards an NHI dispensation.
Annexures
Annex I: Sample Generic Equipment Specification: Infant Incubator
NB: The specification is intended to indicate the range of issues addressed as well as the level of detail, clarity of language and formatting/layout typically associated with a good technical specification; however, since this is being used for illustrative purposes, many clauses have been omitted for the sake of brevity.
1. APPLICABLE DOCUMENTS
The specification should be read in conjunction with the “Technical and Environmental Data Sheet”, and all goods offered must conform to the details specified in it and be able to function in the prevailing conditions described.
2. REQUIREMENTS
2.1 GENERAL DESCRIPTION
To supply: ONE unit to provide a suitable environment conducive for nursing ill, premature, and underweight babies.
2.2 OPERATIONAL REQUIREMENTS
Note: supplier to complete “Reply” and “Remarks” sections.
2.2.1 There shall be a trolley base with four swivel wheels, at least two lockable.
2.2.2 The incubator shall fit securely onto the trolley.
2.2.3 The incubator base shall house the power compartment, fan and humidifier tank.
2.2.4 The infant compartment shall have a base, mounted above the humidifier tank and fan, which is large enough to allow the unimpeded handling of the infant. Base shall have smooth, easy to clean surfaces.
2.2.5 The baby tray shall be mounted on the infant compartment base and shall be tiltable.
…
2.2.8 The infant compartment shall have a transparent canopy that forms four sides and the roof.
2.2.9 The canopy shall be hinged along one side so that it can be swung up to provide free access to the bed.
…
2.2.15 The air shall be drawn into the incubator through an easily removable bacteria filter capable of removing, with an efficiency of 99%, particles of the size down to 0.5 micron diameter.
2.2.16 The air shall be circulated by means of a fan.
2.2.17 The circulated air shall maintain slight positive pressure in the infant compartment such that enough stale air escapes from the hood to prevent an undesirable and dangerous carbon dioxide accumulation inside blood.
2.2.18 The hood shall have inlet holes for access by oxygen and feeding tubes.
…
2.2.21 There will be an air temperature sensor mounted on the inside of the canopy.
2.2.22 The incubator shall be equipped with heating elements of the totally enclosed metal-clad type and a thermostat capable of controlling the temperature in the infant compartment over a specific temperature range.
2.2.23 The incubator shall be equipped with a reliable pre-set high-temperature cut-out that operates completely independently from the thermostat and that disconnects the heating circuit from the electricity supply if, as a result of heating from any source (including direct sunlight or nearby heaters), the temperature in the infant compartment exceeds 39˚C. Any relay forming part of this circuit shall be arranged to be fail-safe.
2.2.24 Temperature range of 34-39 degrees Celsius, in increments of 0.1 degree.
2.2.26 The airflow alarm shall be activated if the airflow is obstructed (due to fan failure or total air circulation failure). The activation of the alarm shall cause a cut off of the heating elements. It shall be mains operated audible and visual.
2.2.27 The high temperature alarm shall be activated if air temperature in the canopy exceeds 39˚C. It shall be mains operated audible and visual.
2.2.28 The power failure alarm shall give warning of any interruption of the electric power supply to the incubator. The alarm shall be operated from a battery of the nickel cadmium type that is housed in the power compartment and is continuously trickle-charged when the power is switched on. The alarm shall be audible and visual.
2.2.29 Single-phase power supply of 220-240 Vac, 50Hz.
2.2.30 To be able to withstand mains supply voltage fluctuations of +/- 10%, and mains supply frequency fluctuations of +/- 10%.
2.2.31 The incubator shall be equipped with a 3-metre non-kinking type flexible mains lead, fitted with a three pin (square)13A plug. The mains connector to be detachable locking type.
2.2.32 The humidifier tank will consist of a water reservoir, water inlet port, and water outlet drain constructed in such a way that once drained a residue puddle of water cannot remain in the reservoir.
2.3 PHYSICAL CHARACTERISTICS
2.3.1 The trolley to be of metallic tubular frame of such dimensions and wall thickness as to give acceptable strength and rigidity. It shall have a polyester powder coating finish.
2.3.2 The casters will be of a minimum size of 100mm.
2.3.3 The incubator base shall be of metal construction with a polyester powder coating finish.
2.3.4 The power compartment shall be of metal and so designed that the mechanical and electrical equipment within it is adequately protected against mechanical damage and the ingress iof water and cleaning fluids.
…
2.3.7 The bed tray and support shall be of corrosion-resistant material.
…
2.3.10 It should be possible to fully dismantle the equipment for cleaning purposes; and all parts will be easily cleaned.
2.4 SAFETY FEATURES
2.4.1 The unit must be manufactured to conform to the IEC safety standard 60101 for medical electrical equipment 2.4.2 Safety Classification: Type B.
3. ACCESSORIES AND CONSUMABLES
3.1 The trolley base to contain a storage compartment with latching doors.
3.2 An IV pole shall be attached to the trolley.
3.3 An oxygen cylinder holder shall be attached to the trolley.
…
3.6 Supply all necessaries for the unit to function as described.
3.7 A list of each accessory and its cost must be stated.
3.8 State all consumables necessary for the unit to function for two years.
3.9 A list of each consumable and its cost must be stated.
4. DOCUMENTATION
4.1 Supply an operating manual in English for the machine.
4.2 Supply a service manual in English for the machine.
4.3 Supply a list of recommended spare parts required for the maintenance of the machine,
5. SPARE PARTS
5.1 Supply a set of only the recommended essential spare parts for 24 months for maintenance and repair.
5.2 A list of each part and its price must be attached to this bid.
6. DELIVERY
6.3 The cost of freighting the goods by sea and road (alt: air and road) to health facility X must be stated.
7. INSTALLATION/COMMISSIONING/TRAINING
7.1 Full assembly and commissioning instructions must be provided for assembly and commissioning by the client, in a written format and as a video if available.
7.2 The cost of commissioning by the supplier or representative must be stated.
7.3 State the cost of the supplier or representative undertaking training and providing written guidelines:
- in operation – for users;
- in care and cleaning – for users;
- in PPM – for maintenance technicians; and
- in repair – for maintenance technicians
8. WARRANTY
8.1 A guarantee period must be stated (a minimum of 12 months from the date of commissioning).
9. AFTER-SALES SUPPORT
9.1 After-sales support must be available, with maintenance capabilities and facilities, and spare parts stock holdings.
9.2 Details of the availability and location of spare parts must be stated.
9.3 Details of the availability and location of maintenance facilities must be stated.
9.4 The cost of the annual maintenance contract must be stated, detailing the range/scope of such maintenance work.
10. SUMMARY OF PRICES (detailed as follows: total prices, showing options and alternatives):
1. Basic unit.
2. Accessories as detailed.
3. Optional accessories.
4. Consumables.
5. Documentation.
6. Spare parts for maintenance and repair for 24 months.
7. Crating, delivery, insurance.
8. Commissioning, training.
9. Annual maintenance contract.
Annex II: Healthcare technology standards and indicators
Standards
The following standards are suggested in alignment with some of the key building blocks of an HTM System.
HT Planning (S1)
There is a system in place which ensures that health technologies are properly planned and provided for in response to disease burden profiles and healthcare needs of populations served, in alignment with service delivery needs and in compliance with organisational and financial constraints.
HT Inventory (S2)
There is a system in place which ensures that appropriate and adequate information on health technology/ medical device assets is maintained, and that this information is validated, regularly updated and accessible to HTM-related decision-making structures and processes at all levels of healthcare delivery.
HT Budgeting and financing (S3)
There is a system in place which ensures that health technologies and related expenditures are properly budgeted for and appropriately and sustainably financed, in accordance with prescribed regulations and organisational policies.
HT Requisition (S4)
There is a system in place which ensures that health technology requests at all levels are properly initiated, screened and collated, according to established criteria and in compliance with specified formats and processes.
HT Procurement (S5)
There is a system in place which ensures that health technology requests, if properly drafted and considered, are translated into a transparent, criteria-driven process that ensures the acquisition of the best technology solution with due consideration of organisational needs and preferences and related criteria.
HT Receipt, testing, installation & commissioning (S6)
There is a system in place which ensures that medical equipment, as part of the procurement process, is properly received, tested, installed and commissioned prior to use in the healthcare environment, in accordance with laid-down procedures and accepted good practice. Pre-installation site preparation or modification may be required.
There is a system in place which ensures that both medical equipment users and maintenance personnel receive appropriate and ongoing training and skill development, so that healthcare interventions and procedures requiring medical equipment, devices and/or instruments are delivered safely and effectively.
HT operation (S8)
There is a system in place which ensures that health technologies are utilised and managed optimally so as to achieve maximal benefit in terms of health outcomes, as part of quality healthcare delivery.
HT maintenance, risk management and safety (S9)
There is a maintenance and risk management system in place that, in keeping with international and national standards and practice, ensures safety of staff and patients.
Indicators
Core indicators (ex WHO)
I. Inputs and Processes
1. Procurement budget
Description:
Total budget spent on new acquisitions in relation to total current asset replacement value (%).
Rationale:
A sufficient budget allocation is necessary in order to replace outdated assets and keep pace with rapid developments in new medical procedures and changing trends in technology. The total budget combines new acquisitions (additions to the existing asset base) and funds for the replacement of outdated and obsolete assets. The total budget combines funding from all sources. Benchmark/Target: 10% or higher.
Measurement:
Evaluation of budgets, at national, sub-national or facility level, extracting the budget spent on new acquisitions, in relation to the total asset replacement value.
Effort level:
Low to medium.
Numerator:
Total budget spent on new acquisitions per annum.
Denominator:
Total current asset replacement value.
Data collection methods/sources:
Statistics to be provided by the health institutions administration, technical department and/or MoH.
Periodicity:
Annual.
2. Maintenance budget
Description:
Total combined budget spent on maintenance and training in relation to total current asset replacement value (%).
Rationale:
Proper maintenance of assets and training of support staff in the use and maintenance of assets is a prerequisite for effective asset management. The budget spent on maintenance and training has to be in a sound relation to the total asset replacement value. Benchmark/Target: 6% or higher.
Measurement:
Evaluation of budgets, at national, sub-national or facility level, extracting the budget spent on maintenance and training, in relation to the total asset replacement value.
Effort level:
Low to medium.
Numerator:
Total budget spent on maintenance and training.
Denominator:
Total current asset replacement value.
Data collection methods/sources:
Statistics to be provided by the health institutions administration, technical department and/or MoH.
Periodicity:
Annual.
3. Workforce
Description:
Number of trained technical support staff related to number of devices.
Rationale:
Only with a sufficient number of qualified managerial, engineering and technical staff can adequate, high-quality health infrastructure and technology management, and thus high quality healthcare, be sustained. Benchmark: ratio in countries with high-quality CE services.
Measurement:
The number of trained technical support staff per 1 000 medical devices.
Effort level:
Low.
Numerator:
The number of trained technical support (CE) staff.
Denominator: \
Total number of medical devices supported.
Data collection methods/sources:
Statistics to be provided by the health institutions administration, technical department and/or MoH.
Periodicity:
Annual.
II. Outputs
Asset quality
Description:
Proportion of assets ready for service delivery in a reliable and safe manner (%).
Rationale:
At any given time, all assets should be in working and safe condition and be used to their full capacity. There should be no defective, unsafe or outdated assets. Benchmark: 100%.
Measurement:
Inspection (audit) of all assets in regular intervals and maintaining a checklist indicating working condition, safety and usage.
Effort level:
Low to medium.
Numerator:
Number of assets ready for service delivery - i.e. reliable and safe.
Denominator:
Total number of assets.
Data collection methods/sources:
Statistics to be provided by the health institutions administration, technical department and/or MoH.
Periodicity:
Annual.
5. Asset downtime
Description:
Average downtime of assets following breakdown or failure (days).
Rationale:
The period of time that it takes the service department to restore defective assets in health facilities to their original working state should be kept to a reasonable and feasible minimum. Benchmark/Target: Minimum period of time (days).
Measurement:
Statistics on downtime of all equipment (by major category/type).
Effort level:
Low.
Numerator:
Average downtime of assets following breakdown or failure (in days).
Data collection methods/sources:
Statistics to be provided by the health institutions administration, technical department and/or MoH.
Periodicity:
Annual.
III. Outcomes
6. Service access
Description:
Percentage of HIT-related patient referrals.
Rationale:
Unnecessary referrals to other, normally higher-level facilities, as a result of asset-related problems, delay the treatment of patients and cause additional costs, thus negatively reflecting on the quality of healthcare. Benchmark/Target: No referrals (0%).
Measurement:
Statistics on the total number of patients and the number of health infrastructure- and technology-related referrals.
Effort level:
Low.
Numerator:
Number of patients being referred to another facility due to asset problems (lack of equipment or consumables, equipment out of order, etc.).
Denominator:
Total number of patients making use of facility (as either out- or inpatients).
Data collection methods/sources:
Statistics to be provided by the health institutions, healthcare providers and MoH.
Periodicity:
Annual.
7. Service delay
Description:
Percentage of patients unduly delayed due to asset failure.
Rationale:
With adequate implementation of proper management processes, patient wait times due to asset failure will be kept to a minimum. Benchmark/Target: No delays (0%).
Measurement:
Statistics on asset failure and the number of patients delayed by these failures.
Effort level:
Medium.
Numerator:
Number of patients with delayed diagnosis or treatment due to asset failure.
Denominator:
Total number of patients making use of facility (as either out- or inpatients).
Data collection methods/sources:
Statistics to be provided by the health centres and MoH.
Periodicity:
Annual.
IV. Impact
8. Patient safety
Description:
Rate of adverse events due to asset failure.
Rationale:
HIT-related incidents and accidents sharply decrease with improving the quality of the technical support services. Asset-related adverse events can affect both staff and patients and must be notified. Benchmark/Target: No adverse events during the period of assessment.
Measurement:
Evaluation of accident and incident reports.
Effort level:
Medium, decreasing to low with the introduction of an accident and incident notification system.
Numerator:
Number of HIT-related accidents and incidents for the institution or level assessed.
Denominator:
Total number of patients making use of facility (as either out- or inpatients).
Data collection methods/sources:
Statistics to be provided by the health centres and MoH. Statistics from the accident and incident reporting system. Statistics from the WHO safe surgery checklist.
Periodicity:
Annual.
9. Staff satisfaction
Description:
Degree of satisfaction of health workers with asset performance.
Rationale:
Staff satisfaction is to a large extent related to the positive or negative contribution of asset performance to the effectiveness and efficiency of healthcare delivery. Benchmark/Target: High degree of satisfaction.
Measurement:
Evaluation of questionnaires. A representative sample of health workers expresses their level of satisfaction on a scale of 1-5: (1) Very poor, (2) Poor, (3) Reasonable, (4 ) Good, (5) Excellent.
Effort level:
Medium.
Numerator:
Satisfaction score (average and range) as per agreed grading system.
Data collection methods/sources:
Questionnaires to be answered by a representative sample of health workers in the health institutions.
Periodicity:
Annual
10. Financial risk
Description:
Proportion of assets in good working condition, but not used.
Rationale:
With appropriate management, all assets should be used up to their full capacity. In cases were assets are in good working condition, but not fully utilised, this is seen as a financial waste. Benchmark/Target: 0%.
Measurement:
Inspection of all assets at regular intervals and maintaining a checklist indicating their use.
Effort level:
Low.
Numerator:
Number of assets not used (or used to less than 50% of expected capability), though in good working condition.
Denominator:
Total number of assets.
Data collection methods/sources:
Statistics to be provided by the health institution administration, technical department and/or MoH.
Periodicity:
Annual.
References and selected bibliography
1. Banta D, Luce B (1993). Health Care Technology and its Assessment: An International Perspective. Oxford University Press.
2. Department of Health, Republic of South Africa (2000a). A Framework for Health Technology Policies.
3. Department of Health, Republic of South Africa (2004). Health Technology Assessment: Discussion Document on a Strategy for the Future. Report of Interim Steering Committee on Health Technology Assessment.
4. Department of Health, Republic of South Africa (2005). National Health Technology Management Policy (draft).
5. Federal Ministry of Health, Republic of Sudan (2011). Health Technology Management Policy (Medical Devices)
6. Locke GP (2000). Safety and Risk Management. Course Notes, Healthcare Technology Management (HTM) Programme, University of Cape Town.
7. Ministry of Health, Republic of Uganda (2009). National Medical Equipment Policy (4th edition).
8. Nunziata E (2003). Workshops overview. Basic Principles and Implementation Strategy.
9. Nunziata E, Poluta M (2006). Health Technology Unit: Framework for Establishment of Regional Workshops. Consultancy Report.
10. Nunziata E, Poluta M (2006). Medical Device Acquisition and Procurement Policy: Draft and Background Document. Consultancy Report.
11. Nunziata E, Poluta M (2012). Procedures, Standards and Norms for Healthcare Technology Lifecycle Management. Consultancy Report.
12. Panerai R, Mohr B (1989). HTA Methodologies for Developing Countries. PAHO.
13. Poluta M (2013). Course Notes (various), Healthcare Technology Management (HTM) Programme, University of Cape Town.
14. Ridgway M, Atles LR, Subhan A ,Reducing Equipment Downtime - A New Line of Attack. (2009). J of Clin Eng, Oct/Dec issue, pp 200-4.
15. Rosen R, Gabbay J (1999). Linking health technology assessment to practice. BMJ vol. 319.
16. Stevens A, Milne R, Lilford R, Gabbay J (1999). Keeping pace with new technologies: systems needed to identify and evaluate them. BMJ vol. 319, Nov.
17. Temple-Bird C (2000). Practical Steps for Developing Health Care Technology Policy. Institute of Development Studies, Brighton.
18. Wang B, Fedele J, Pridgen B et al (2010). Evidence-Based Maintenance - Part I: Measuring Maintenance Effectiveness with Failure Codes. J of Clin Eng, Jul/Sep issue pp 132-144.
19. WHO (1989). Manpower Development for a Health Care Technical Service – Report of WHO Interregional Meeting, Campinas, Brazil.
20. WHO (1997). Guidelines for Healthcare Equipment Donations.
21. WHO (1998). Final Report of the WHO Consultation on Physical Infrastructure and Technology.
22. WHO (2001). Report of Technical Consultation on Effective Coverage in Health Systems WHO/EIP/OSD/10.01.
23. WHO (2002). Safe Medical Devices. Aide-Memoire for National Medical Device Administrations.
24. WHO (2008a). Strengthening Healthcare Infrastructure and Technology Management for Optimized Health Service Delivery. Luxembourg – WHO Project Technical Document.
25. WHO (2008b). Measuring Health Systems Strengthening and Trends: A Toolkit for Countries.
26. WHO (2010a). A Framework for National Health Policies, Strategies and Plans. Consultation Document (draft 3).
27. WHO Collaborating Centre for Essential Technologies in Health (1998). The Essential Technology Package – a decision-support tool for selection and codification of equipment based on identified health interventions and delineated procedures.
28. WHO. Towards a WHO Model List of Essential Medical Devices. Dept. of Essential Health Technologies.
29. WHO/World Bank/GAVI/Global Fund (2009). Monitoring and Evaluation of Health Systems Strengthening – An Operational Framework.
30. WHO-AFRO (1998). Guidelines for the Formulation of a National Equipment Policy.
31. WHO-AFRO (1999). Health Technology Policy in the African Region – Horizon 2010.
32. WHO-EMRO (1997). Resolution EM/RC44/R3 on Appropriate Health Technology.
33. WHO-EMRO (2001a). Eastern Mediterranean Regional Strategy for Appropriate Health Care Technology. No. 2 in Series on Health Care Technology Management.
34. WHO-EMRO (2001b). Health Care Technology Policy Formulation and Implementation. No. 3 in Series on Health Care Technology Management.
35. WHO-EMRO (2001c). Health Care Technology Policy Framework. No. 1 in Series on Health Care Technology Management.
36. Ziken International (2005). “How to Manage” Series for Healthcare Technology. DFID KaR Programme
37. WHO Medical Device Technical Series (2011) includes:
1. WHO EHT Computerised Maintenance Management System Guide
2. WHO EHT Donations Guide
3. WHO EHT HTA Guide
4. WHO EHT Inventory Management Guide
5. WHO EHT Maintenance Guide
6. WHO EHT Needs Assessment for Medical Devices
7. WHO EHT Procurement Process Guide
Available at http://www.who.int/medical_devices/management_use/en/
Please help to expand this page. |